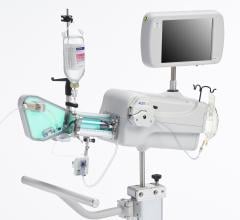
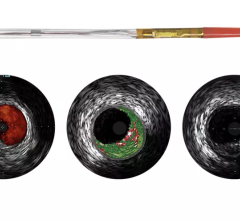
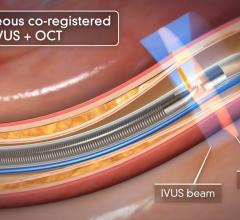
The LipiScan Coronary Imaging System by InfraReDx.
There is considerable interest in being able to clearly identify vulnerable plaque (VP) and research efforts are intensifying. Several invasive imaging technologies claim to detect VP, and while the link between VP identification, plaque rupture and clinical events has yet to be firmly established, progress is being made in the research.
“Basically it’s a plaque that puts the patient at risk for future cardiovascular events; especially, sudden cardiac death, acute myocardial infarction or unstable angina,” said Gregg W. Stone, M.D., professor of medicine at Columbia University College of Physicians and Surgeons, director of cardiovascular research and education at the Center for Interventional Vascular Therapy, New York-Presbyterian Hospital/Columbia University Medical Center.
Sergio Waxman, M.D., and colleagues describe VP as “arterial segments and plaques in which an intraluminal thrombus develops, leading on occasion to the clinical events of acute coronary syndromes (ACS). These segments, which constitute relatively small regions within diffusely diseased vessels, have been termed vulnerable plaques, high-risk plaques or thrombus-prone plaques.”1 According to the authors, three processes may lead to occlusive thrombosis: rupture, erosion and the calcified nodule. The most common type of lesion associated with plaque rupture is the thin-cap fibroatheroma (TCFA), which is thought to account for 60-70 percent of coronary events. Although TCFAs are the most common type of vulnerable plaque, not all TCFAs will rupture. A large lipid core is a major component of TCFA. Eroded plaques may account for 30-40 percent of sudden coronary deaths.
“Vulnerable plaque is probably the No. 1 unmet need to be able to detect and then prophylactically treat for patients with cardiovascular disease,” Dr. Stone said. “Right now you’ve got more than 750,000 people each year dying of heart attacks and 250,000 having strokes. And, for most of them, we can’t prevent them.”
Dr. Stone said the tools exist to identify these blockages using CT and invasive catheters, but cardiologists need to prove that seeing these blockages actually puts a patient at risk. He said therapies also need to be tested to try and prevent complications.
Patients most at risk for VP
It's a challenge to determine ahead of time, which patients are most at risk for vulnerable plaque. “That goal is 'the Holy Grail,' because there is no accepted way to identify patients with vulnerable plaque,” Dr. Stone said. “There is a whole host of invasive and noninvasive imaging modalities that investigators are working on to validate being predictive of vulnerable plaque rupture, but none of those studies are complete.”
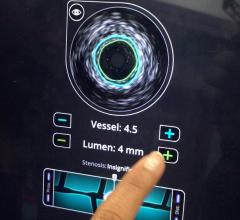
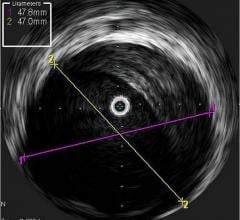
Dr. Stone said of the invasive imaging technologies used to detect vulnerable plaque, intravascular ultrasound (IVUS) is not specific enough. “It just shows plaque everywhere,” he said. “But it doesn't differentiate which plaques are at risk.” He said other modalities include virtual histology, chemography to examine a lipid rich core, intravascular MRI, OCT (a light-based imaging technique that can identify thin caps), vasa vasorum imaging and thermography.
“There's a whole host of techniques that have been developed to identify so e of the characteristics of what we think a vulnerable plaque is, but what has not yet been proven is that if that tests positive then the patient has a two times risk of heart attack, or five times higher risk or 10 times higher risk,” Dr. Stone said. “You have to prove it.”
He said his institution just ran the first natural history study to see if any of these techniques work. The PROSPECT study used virtual ultrasound and histology, but he said it is still in the follow-up phase to see if detecting abnormal plaque based on virtual histology can detect patients with events.
Recently approved technologies
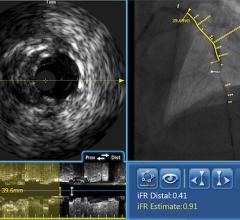
Of the invasive imaging technologies, Dr. Stone said the one that is more established is the LipiScan Coronary Imaging System by InfraReDx, which the FDA cleared earlier this year. The technology uses spectroscopy, where near-infrared light is shined on tissue and the reflected wavelengths are analyzed to identify its chemical makeup. James E. Muller, M.D., cardiologist, co-founder, president and CEO of InfraReDx Inc. resigned from his position as professor of medicine at Harvard Medical School to bring the device to market. He said the device offers a lipid burden index, which is a summary of all of the lipid core signals in a particular artery.
“This is a new tool. Its potential utility is still not clear. It may sound a bit grandiose, but I think it's a bit like the electrocardiogram, first described in 1905. It wasn’t until 15 years later in 1920 that Pardee recognized that ST segment elevation in the ECG was a sign of heart attack. So, I think we're in that early stage now.”
The company believes the device will assist stenting decisions and in patient management decisions, including the length of the area to be stented.
With the LipiScan, it is now possible to go in and say a patient does not have any more stenoses and they don't have any more lipid core plaques, Dr. Muller said. He explained the long-term use requires a natural history study where patients are followed over time to see if a yellow spot on the chemogram actually predicts a clinical event or if a certain plaque will grow and become more stenotic. He said InfraReDx is in the process of conducting such studies.
“A long-term hope for us, of course, is that we can find the plaques that will cause a second heart attack and the interventional cardiologist can treat them, and that way the statement that ‘interventional cardiologists are only good for relieving symptoms’ can be changed,” Dr. Muller said.
Brij Maini, M.D., FACC, co-chair, cardiovascular research, Pinnacle Health in Harrisburg, PA, used the LipiScan system is 12-14 cases as of September.
“Eventually it will help treat these patients,” he said. “The future clinical trials will help us determine appropriate treatment decisions. Do we pursue high statin therapy? Do we stent them? At this point in time, those questions have not been answered.”
Dr. Maini said the LipiScan is getting medicine closer to the truth about vulnerable plaque. “It’s a very user friendly platform,” he said. “We will know more about the natural history of vulnerable plaque when there are treatment options that become available.”
As for comparing this technology to VH IVUS, in Dr. Maini’s opinion, “That is the billion dollar question. Nobody knows the answer to that. Each of these technologies has done their own histogram testing. Each claims that they are as sensitive as you can get, but there has been no head- to-head comparison. We are in the process of doing a head-to-head comparison, but the study is not done yet.”
Volcano Corp. produces an IVUS catheter-based system that images diseased vessels from inside the artery. IVUS provides detailed and accurate measurements of lumen and vessel size, plaque area and volume and the location of key anatomical landmarks. Volcano said its proprietary VH IVUS technology helps differentiate the four plaque types: fibrous, fibro-fatty, necrotic core and dense calcium.”2
“The Volcano Virtual Histology System is the most advanced technology available today that allows us to assess vulnerable plaque,” said Joseph Puma, M.D., Lenox Hill Heart and Vascular Institute, department of interventional cardiology, Lenox Hill Hospital, NY. “We simply do not have any noninvasive ways to reliably assess vulnerable plaque and invasively, the only way to assess vulnerable plaque is through the virtual histology software intravascular ultrasound. It’s a very sophisticated software program that augments grayscale intravascular ultrasound to create many more slices to create a computer generated representation of the composition of the plaque.”
Dr. Puma said pundits of virtual histology say more clinical validation is needed. He said there is a registry with thousands of patients funded by Volcano and more than 100 investigators nationwide have enrolled patients to collect data and assess the clinical diagnosis with virtual histology findings. He said the PROSPECT trial is a randomized, controlled trial using virtual histology findings to help make treatment decisions. Dr. Puma said it is now in follow-up and within six months registry data is expected to be published, and in one to two years he hopes clinical trial data will help further validate the technology.
He said patients who should be targeted for VH IVUS include those who have a crescendo pattern of angina or unstable angina where the high-grade stenosis is not the cause the pain.
“It’s the stenosis that is usually nonobstructed angiographically that is the problem but pathologically we know has had multiple ruptures that causes this pattern of angina,” Dr. Puma said. “And, the only technology that allows us to identify that is virtual histology.”
The second group of patients would be those with acute coronary syndrome who have a more advanced stage of unstable angina who may have already had a positive biomarker - a troponin, or CKMB bump or significant ST segment deviation.
Dr. Puma said in comparing VH IVUS to LipiScan, “the InfraReDx system is not in widespread clinical use, not easy to use, and most labs do not have that system. That system still has a ways to go... If you're going to use IVUS and if you use it with the Volcano system, it’s very important to use VH, since it doesn’t cost any more, it doesn’t take more time and it’s a simple software application to the area you’ve already acquired.” <
1. Waxman, S, Ishibashi, F, and Caplan, JD. Rationale and use of near-infrared spectroscopy for detection of lipid-rich and vulnerable plaques. J Nuclear Cardiology 2007 Sept-Oct; 14(5): 719-728.
2. Volcano Corp., www.volcanotherapeutics.com/products/ivus-imaging/index.asp, accessed Sept. 12, 2008.




 September 18, 2025
September 18, 2025