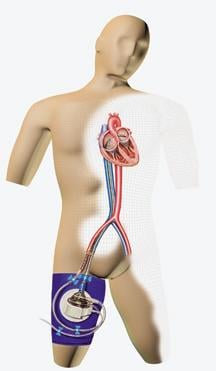
June 25, 2008 - Cardiologists completed the 1,300th worldwide procedure utilizing the TandemHeart System, said manufacturer CardiacAssist, marking the increased acceptance of the device and use by cardiologists and surgeons.
The TandemHeart System assists heart failure patients survive both heart attacks and high risk procedures, while providing versatility to physicians in choosing the time, place and type of treatments. The system has a high net flow rate of up to 5 lpm in the Cath Lab or up to 8 lpm in the OR as well as its flexibility and rapid deployment potential. There are a variety of FDA cleared devices that provide extracorporeal circulatory support, but all others offer less than half the net flow and hemodynamic support of the TandemHeart System.
The TandemHeart System is now used in over 100 heart centers across the U.S. Ten prestigious heart centers were recently active in helping CardiacAssist, Inc. surpass the 1,300th procedure for its TandemHeart System. These 10 heart centers include:
-- Brigham & Women's Hospital in Boston, MA
-- Cleveland Clinic in Cleveland, OH
-- Hospital of the University of Pennsylvania in Philadelphia, PA.
-- Lahey Clinic in Burlington, MA
-- Lenox Hill in New York City, NY
-- Mayo Clinic in Rochester, MN
-- Mt. Sinai in New York City, NY
-- Sacred Heart Medical Center in Spokane, WA
-- St. Luke's Hospital in Kansas City, MO
-- Texas Heart Institute in Houston, TX
The TandemHeart System is fully reimbursed by Medicare under existing DRG codes. The device can be placed rapidly in the cath lab or operating room, providing effective, reliable, temporary circulatory support for critically ill patients.
For more information: www.cardiacassist.com


 June 19, 2024
June 19, 2024 









