June 2, 2008 - Abiomed Inc. received 510(k) clearance from FDA for Impella 2.5 Device Cardiac Assist Device, allowing Abiomed to sell the device in the U.S.
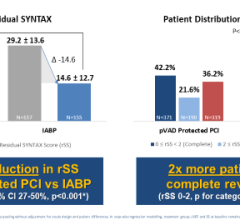
The Impella 2.5 is cleared for use under the 510(k) for partial circulatory support for periods up to six hours. The intra-aortic balloon pump (IABP) also has 510(k) clearance and approximately 110,000 are used each year in the United States. Abiomed is currently conducting two U.S. pivotal studies comparing the Impella 2.5 to the IABP.
The Impella 2.5 is inserted percutaneously in the catheterization laboratory via the femoral artery into the left ventricle. Up to 2.5 liters of blood per minute are delivered by the pump from the left ventricle into the ascending aorta, providing the heart with active support in critical situations.
"FDA clearance of the Abiomed Impella 2.5 represents the next step in enabling heart recovery for patients in the U.S. and will likely change the standard of care in the catheterization lab," said Abiomed Chairman, CEO and President Michael R. Minogue. "The device seamlessly provides immediate, minimally invasive circulatory support for critical patients."
The Impella 2.5 is now approved in more than 40 countries, including in Europe for up to seven days of support under the CE Mark.
For more information: www.abiomed.com

 August 14, 2023
August 14, 2023