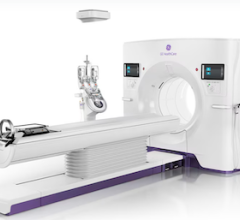
May 13, 2008 – The FDA cleared GE Healthcare’s new LightSpeed CT750 HD, said to be the world’s first high-definition CT scanner that produces images 100 times faster with up to 33 percent greater detail through the body and up to 47 percent greater detail in the heart.
CT750 HD reduces dose by up to 50 percent across the entire body and by as much as 83 percent for cardiac scans.
The new CT utilizes Gemstone Spectral Imaging and uses up to 2496 views per rotation (a 2.5x increase) to deliver improved spatial resolution and improved image quality across the entire field of view. Dual energy fast kV switching registers energies at least 165 times faster than Dual Source CT at a .33s rotating speed. It offers 128 slices of unique data per rotation and 101 user selectable energy levels for viewing, reportedly producing faster, clearer images without sacrificing radiation dose reduction.
For more information: www.gehealthcare.com


 February 02, 2026
February 02, 2026