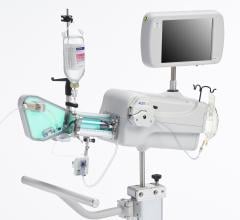
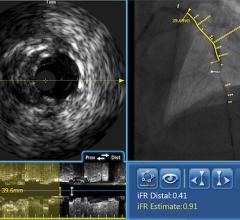
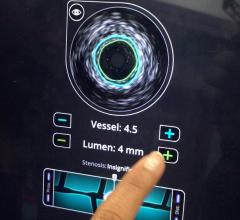
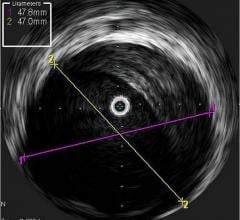
February 7, 2008 - Volcano Corp. said the FDA cleared the s5-Revo and s5-FFR (fractional flow reserve) options, enabling rotational intravascular ultrasound (IVUS) and FFR to operate on the same integrated Volcano s5 Imaging System as Volcano’s previous line of phased array IVUS catheters and functionality.
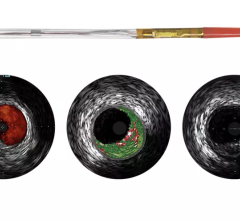
Earlier generation consoles included only one of the three technologies now available on the s5. If a hospital wanted to equip a new lab with all three technologies, they would have to acquire three separate consoles, each with a different measurement modality, training requirements and data storage protocols, according to the company. The Volcano s5 can now accommodate the three primary intravascular diagnostic tools in regular use by cardiologists today (high frequency rotational IVUS, digital IVUS and pressure-based FFR guidewires) on a single platform.
In preparation for the s5-Revo and s5-FFR launch, Volcano has increased its training offering to incorporate additional site education on all three of these primary technologies.
The first installations of the Volcano s5-Revo and s5-FFR Options under a limited market release strategy will commence shortly with a full market release expected in the first half of 2008. The Volcano s5-Revo and s5-FFR options complement the existing s5 functionality, including ChromaFlo for stent apposition assessment, and VH IVUS plaque composition analysis.
The Volcano s5 Imaging System has been tested in cooperation with GE, Philips, Siemens and Toshiba for safety and compatibility when used with their current lines of x-ray systems and equipment. The Volcano s5 has also been tested for compatibility with eleven different DICOM providers, and for Worklist compatibility with image storage in the same patient “jacket” as the angiogram.
For more information: www.volcanocorp.com


 September 18, 2025
September 18, 2025