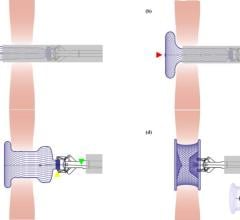
January 30, 2008 - Aporo Biomedical signed an exclusive global licensing agreement with mNEMOSCIENCE GmbH for its proprietary biodegradable shape memory polymers (BIO-SMP), enabling Aporo to offer devices with biodegradable closure features for treating structural heart diseases such as PFO and ASD.
Initially, Aporo will use these materials to treat Patent Foramen Ovale (PFO). In the future, the company will use the polymers in devices to treat Atrial Septal Defect (ASD), another structural heart disease, and for vascular closure after catheter-based interventional procedures.
Although minimally invasive catheter-based procedures are relatively short and can provide significant benefits to the patient, there is a growing desire among clinicians to avoid the potential complications and disadvantages from permanently implanting a large device in an otherwise healthy heart. Aporo Biomedical plans to address this concern by delivering a biodegradable device that closes the PFO without leaving a permanent implant.
For more information: www.aporobiomedical.com


 June 20, 2024
June 20, 2024