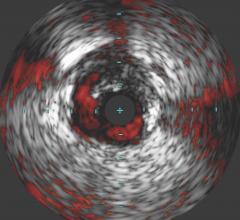
April 4, 2007 — Volcano Corp. and ev3 Inc. have announced they have entered into a joint marketing and distribution agreement.
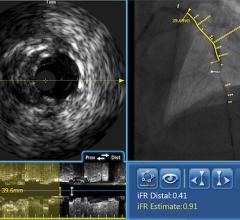
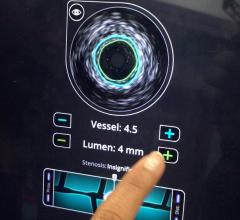
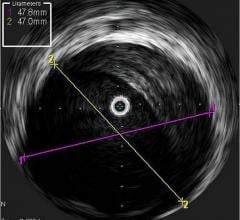
Under the terms of the agreement, Volcano will have the opportunity to sell ev3’s SpiderFX Embolic Protection Device in conjunction with its Intravascular Ultrasound (IVUS) and Functional Measurement (FM) devices for use in saphenous vein grafts (SVGs) in the U.S. The SpiderFX is FDA cleared for use during the endovascular treatment of both SVGs and carotid arteries.


 November 07, 2024
November 07, 2024