May 20, 2008 – Volcano paid $12 million in cash at the closing for the acquisition of Novelis Inc., a privately-held company that develops imaging products to use in crossing chronic total occlusions (CTOs), enabling Volcano to incorporate Novelis’ technology into its s5i integrated IVUS platform.
Under the terms of the agreement, Volcano paid approximately $12 million in cash at the closing and will may make an additional cash payment of $3 million based on the achievement of a specific regulatory milestone.
Novelis’ Forward-Looking IVUS technology platform is expected to build upon Volcano’s existing suite of products and further enhance Volcano’s in the field of interventional medicine by enabling forward-looking IVUS and associated therapies in the interventional cardiology market.
Novelis’ offering consists of a forward-looking manually steered imaging system comprised of a laptop-sized console with advanced custom software and single-use catheters both with and without RF ablation. The system and accessories are not approved for human use at this time and have not been submitted to FDA for regulatory clearance. Volcano expects to add the Novelis products and capability onto its s5i multimodality integrated platform/hub.
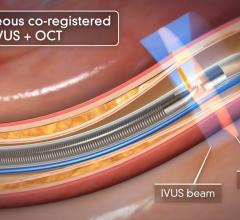
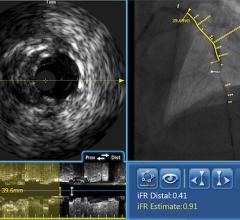
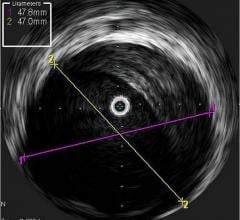
Novelis’ core product line is based on Forward-Looking Intravascular Ultrasound (FLIVUS) technology. The product line includes an image-guided crossing catheter that combines visualization, steerability and RF tissue ablation, which is designed to permit interventional cardiologists to safely cross chronic total occlusions in the coronary and peripheral arteries, and a forward-looking IVUS catheter (imaging-only version) to facilitate current guidewire-based CTO crossing techniques as well as other potential product offerings with possible applications outside of vascular medicine. This platform technology may have applications for numerous minimally invasive procedures, including plaque modification in the coronary or peripheral arteries, guidance in structural heart procedures such as cardiac ablation guidance and therapy, breast biopsy guidance and therapy, and orthopedic (spine) guidance and therapy.
“Novelis’ proprietary technology has potential applications for a number of minimally invasive diagnostic and therapeutic applications in the coronary and peripheral arteries, including the treatment of CTOs. It is estimated there are currently over 200,000 CTOs performed in the U.S., Europe and Japan each year. These procedures, which remain one of the most challenging issues faced by interventionalists today, often fail or take hours to successfully complete due to inadequate visualization. Furthermore, due to the lack of adequate tools for CTO crossing, many interventionalists simply refer patients with CTOs to highly invasive and costly coronary artery bypass grafting (CABG) procedures. The products under development by Novelis have the potential to shorten procedure times and minimize complications plus dramatically add to the total number of procedures being performed – enabling patients with CTOs to be treated in the cath lab rather than in the surgery suite. At the estimated procedure volume of over 200,000 cases per year – and factoring in the potential for market expansion - the total market potential for Novelis’ CTO products is estimated at over $500 million,” he added.
“We believe the regulatory path is fairly straightforward in all major geographies and that this offering will leverage our strong presence in the cath lab through our sales and distribution programs. Given our sales team’s role as advisor to interventionalists in ultrasound image interpretation, we believe this is an absolutely perfect match with our sales and marketing capabilities. We are also very excited to explore the potential for this technology platform in other large expanding markets like ablations for atrial fibrillation and spine therapy,” Huennekens concluded.
“CTOs continue to be a challenge for even the very experienced interventionalist. Current angiographic tools are simply inadequate to guide CTO crossing,” commented Gary Mintz, M.D., chief medical officer, Cardiovascular Research Foundation, medical director and editor-in-chief, TCTMD.com and advisor to Volcano. “The next step to improve success rates will come in the form of intravascular image guided therapies such as this truly unique ‘forward looking’ approach to CTO imaging,” he added. “Many technologies have been tried; but to date, none have been particularly successful. Novelis is the first forward-looking technology that I have seen that makes sense and seems to have a strong likelihood of providing a viable solution for guiding CTO recanalization.”
The company expects to file for appropriate U.S. and international approvals on the first of several devices during 2009 and begin commercialization of a stand-alone imaging console in the second half of 2009 in the U.S. and Europe. Volcano expects a majority of the purchase price to be written off immediately as in process research and development and to incur ongoing development costs in the range of $300,000 to $400,000 per quarter.
For more information: www.volcanocorp.com


 November 07, 2024
November 07, 2024