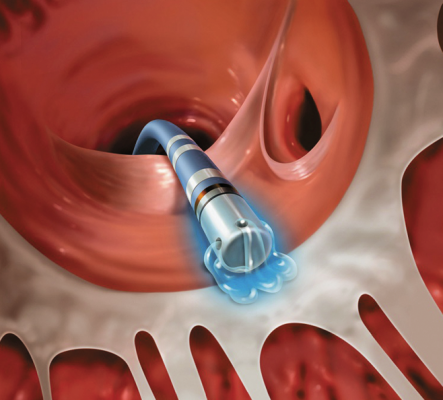
June 17, 2014 — University Hospitals (UH) Case Medical Center is among the first in the country to offer BioSense Webster's ThermoCool SmartTouch catheter, the first catheter approved by the U.S. Food and Drug Administration (FDA) to feature direct contact force technology for the treatment of patients with atrial fibrillation. This novel innovation enables doctors to accurately control the amount of contact force applied to the heart wall during radiofrequency catheter ablation procedures.
“Consistent and stable application of contact force against the heart wall has been demonstrated to have a significant impact on patient outcomes during catheter ablation. This new catheter provides critical contact force information to help confirm that we are applying the intended amount of pressure throughout the procedure so that optimal outcomes can be achieved,” said Mauricio Arruda, M.D., director, clinical electrophysiology and pacing, UH Case Medical Center, and professor of medicine at Case Western Reserve University School of Medicine. “Without this technology, doctors have to estimate the amount of force being applied to the heart wall through other indirect measures that have been shown not to be as effective.”
During a minimally invasive catheter ablation procedure, physicians insert a therapeutic catheter through a small incision in the groin where it is then weaved up to the heart through a blood vessel. Once it reaches the left upper chamber of the heart, the catheter delivers radiofrequency energy to the heart wall to create lesions to block faulty electrical impulses that can cause heart rhythm disorders. Providing doctors with the ability to apply stable contact force during catheter ablation has been shown to improve patient outcomes as poor tissue contact may result in incomplete lesion formation. This can result in the need for additional treatment, and too much contact force may result in tissue injury, which may lead to complications.
One-year results from a clinical trial that studied the safety and effectiveness of the device showed that patients experienced a 74 percent success rate after treatment with the ThermoCool SmartTouch. Importantly, data from the trial showed higher success rates the longer physicians stayed within a targeted contact force range, with one-year results demonstrating an 88 percent success rate when physicians stayed within a targeted range greater than or equal to 85 percent of the time.
For more information: www.uhhospitals.org


 February 06, 2026
February 06, 2026 









