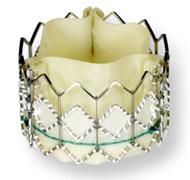
February 25, 2011 – A larger transcatheter aortic heart valve has received the CE mark. The 29 mm version of the Sapien XT valve, from Edwards Lifesciences, promises to expand the number of patients who can be treated.
The valve will be available with the Ascendra transapical delivery system for implantation through a small incision between the ribs. This new offering increases the valve portfolio to three sizes.
"We knew it was important to provide patients with a 29 millimeter valve, and we're pleased we can now help even more patients in need," said Larry L. Wood, Edwards' corporate vice president, transcatheter valve replacement.
The valve builds upon the features of the Edwards Sapien valve, incorporating a tissue leaflet design that is modeled on the company’s surgical aortic tissue valves and a cobalt chromium frame that provides improved radial strength. The Sapien XT valve has been used widely by clinicians throughout Europe since its commercial launch in the region in 2010, and is an investigational device in the United States that is being studied in The PARTNER II Trial, a randomized controlled study.
For more information: www.edwards.com


 December 24, 2025
December 24, 2025 









