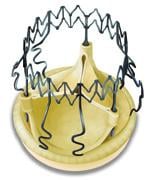
February 1, 2011 – A self-anchoring aortic heart valve has received the CE mark. With the approval, the Perceval S Sutureless biological valve, by the Sorin Group, is now commercially available in Europe.
It is indicated for the rapid and minimally invasive surgical replacement of the aortic valve in patients suffering from aortic stenosis. Aortic stenosis is the most common acquired valvular heart disease in the Western world and its occurrence increases with age. The prognosis is poor for patients with severe symptomatic aortic stenosis. If the patient remains untreated after symptoms appear, the average survival is short – often only two to three years.
The surgical aortic valve has a self-anchoring frame that enables the surgeon to replace the diseased valve without suturing it into place. The valves’ functional component is made of bovine pericardium and is mounted on a super-elastic alloy frame. The system builds on a clinically proven stentless aortic pericardial valve implanted in over 10,000 patients with proven durability and optimal hemodynamics. Clinical results in the first 180 patients show a significant reduction in surgical procedural time for both isolated and complex aortic valve replacement. Aortic cross-clamp times typically reduced by at least 50 percent.
The hemodynamic performance was outstanding with low pressure gradients and large effective orifice areas at one and two years follow-up. To date, 25 cardiac surgery centers throughout Europe have implanted the Perceval S valve in over 500 total patients as part of the pre-commercialization clinical studies.
For more information: www.sorin.com


 February 06, 2026
February 06, 2026 









