Riata and Riata ST leads
Related Content
February 24, 2023 — Biotronik announced that it has received CE approval for the Selectra 3D implant tools to include ...
January 3, 2023 — The market has been studied for the below mentioned-segmentation and regional analysis for North ...
September 20, 2019 — BioTrace Medical Inc. announced the company’s key activities at the 31st annual Transcatheter ...
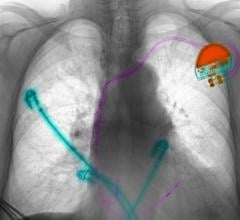
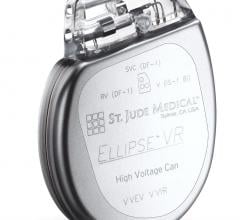
August 5, 2019 — Abbott is recalling the Ellipse Implantable Cardioverter Defibrillators (ICDs) because electrical ...
May 13, 2019 — BioTrace Medical Inc. announced the company’s Tempo Lead has obtained CE Mark certification in Europe for ...
May 6, 2019 — Medtronic has received U.S. Food and Drug Administration (FDA) approval for the Attain Stability Quad MRI ...
May 24, 2018 - Medtronic plc announced results from a research study demonstrating the feasibility of a novel approach ...
May 18, 2018 — Data on the effectiveness of cardiac resynchronization therapy (CRT) in patients with non-left bundle ...
June 20, 2017 — BioTrace Medical Inc. announced that the company’s Tempo Temporary Pacing Lead was featured in an oral ...
Bruce Wilkoff, M.D., director of cardiac pacing and tachyarrhythmia devices at Cleveland Clinic, discusses advancements ...

 February 24, 2023
February 24, 2023