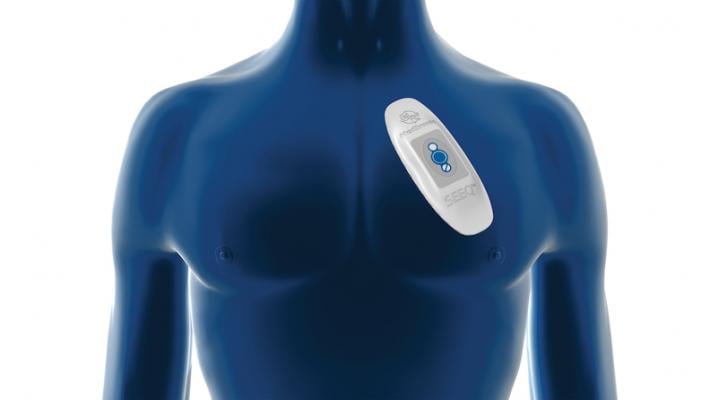
The Medtronic SEEQ mobile cardiac telemetry system, pictured here, and the Icentia CardioSTAT cardiac rhythm monitoring device both found in reased rates of post-operative atrial fibrillation in the SEARCH-AF trial presented at the American Heart Association annual meeting.
November 16, 2020 — Atrial fibrillation (AF), an irregular heartbeat that can increase the likelihood of stroke, was detected up to 10 times more frequently in high risk patients recovering from heart surgery who wore a continuous cardiac monitor for a month, compared to patients who had usual follow-up care following their procedure, according to the SEARCH-AF late breaking study presented today at the 2020 American Heart Association (AHA) Scientific Sessions.
“The incidence of post-operative atrial fibrillation (POAF) after discharge from cardiac surgery is not well defined,” said Subodh Verma, M.D., Ph.D., a cardiac surgeon at St. Michael’s Hospital, University of Toronto in Canada, and one of the lead authors of the study. “Most studies are limited to the hospitalization phase only; studies beyond hospitalization are few. In addition, very little is known about patients who have little to no AF during hospitalization after cardiac surgery, and they are often sent home with no treatment. Therefore, the question of whether the risk of POAF extends after hospitalization remains an important and unanswered question, especially for patients who had no previous history of AF. Quantifying the risk of ongoing POAF after hospitalization is important because many patients have elevated stroke risk scores that increase the need for prompt AF treatment.”
Researchers followed a total of 336 patients treated at eight medical centers across Canada who were recovering from cardiac surgery. The study participants did not have a history of atrial fibrillation prior to surgery and were at-risk for stroke based upon factors including their cardiac health, age and medical history.
The patients in the study were randomly assigned to two groups: 163 study participants underwent 30 days of 24-hour, real time, cardiac rhythm monitoring through an adhesive, patch-based monitor worn on their chest (using the Medtronic SEEQ mobile cardiac telemetry system or the Icentia CardioSTAT cardiac rhythm monitoring device); 173 participants were in the control group and received 30 days of usual care, which did not involve planned cardiac rhythm testing/electrocardiogram assessment unless it was deemed medically necessary. The patients in both groups then had 14 days of continuous cardiac rhythm monitoring approximately six to nine months after their surgery.
The study determined that in the patient group who wore the 24-hour monitoring patch, atrial fibrillation was detected at a rate 10 times higher than in those who received usual care. AF or atrial flutter lasting ≥6 minutes occurred and was detected in 32 (19.6%) patients from the monitoring group and in three (1.7%) patients who received normal care during the 30 days they were followed.
“Our study points to the fact that POAF is not self-limited to hospital stay per se. A significant risk of POAF persists even in those patients without any preoperative or pre-discharge AF. These data may help inform physicians about the importance of surveillance and vigilance in patients at high risk of stroke with respect to monitoring and prompt treatment for AF,” added Verma.
Co-authors of the study include Andrew Ha, M.D.; David Mazer, M.D.; Adrian Quan, M. Phil.; David Latter, M.D.; Bobby Yanagawa, M.D.; Marnee Wilson, N.P.; Terrence Yau, M.D.; Frederic Jacques, M.D.; Craig Brown, M.D.; Rohit Singal, M.D., M.Sc.; Michael Yamashita, M.D.; Tarit Saha, M.D.; Kevin Teoh, M.D.; Buu-khanh Lam, M.D., M.P.H.; Andrew Kosmopoulos, B.H.Sc.; Marc Deyell, M.D., M.Sc.; Chris Cheung, M.D.; Vinay Garg, M.D., M.Sc.; Shira Brodutch, M.Sc.; Hwee Teoh, M.D.; Fei Zuo, M.S., M.P.H.; Kevin Thorpe, M.Math; Peter Juni, M.D.; Deepak L. Bhatt, M.D., M.P.H.; and Atul Verma, M.D. Author disclosures are detailed in the abstract.
The study was funded by the Heart and Stroke Foundation of Canada with unrestricted grants from Bristol Myers Squibb, Pfizer and Boehringer Ingelheim.
Find more AHA late-breaking studies, news and video


 November 14, 2025
November 14, 2025