May 17, 2019 — Biopharmaceutical company CellPoint plans to begin patient recruitment for its Phase 2b cardiovascular imaging study in patients suffering from coronary artery disease using the company's new one-vial Oncardia (ethylenedicysteine-glucosamine) kits. The 60-patient trial study is being conducted in the U.S.
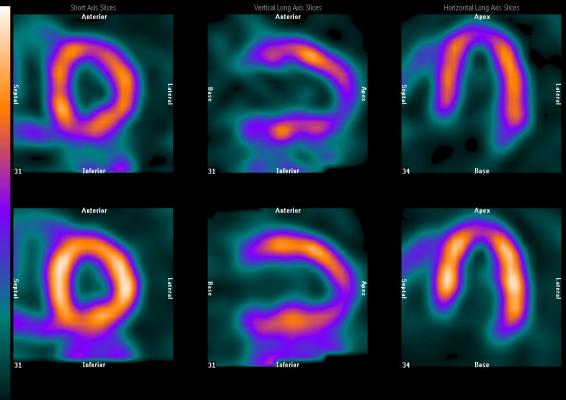
Today, slightly greater than 6 million nuclear cardiology perfusion imaging scans are performed in the U.S. annually. Such procedures, referred to as myocardial perfusion imaging (MPI), are performed using either single photon emission computed tomography (SPECT) or positron emission tomography (PET) camera systems. SPECT MPI procedures represent 98 percent of the total. All MPI procedures, regardless of the choice of radiopharmaceutical agent, are designed to illuminate the healthy perfused part of the heart. A high percentage of the patients are required to undergo a time-consuming rest and separate stress study. While there are several cardiovascular disease indicators that can be looked at with MPI, the predominant focus for such studies is the presence, location and severity of myocardial ischemia following a heart attack.
99mTc-Oncardia is not a perfusion agent. It is being clinically studied as the first functional cardiovascular imaging agent that is target-specific for myocardial ischemia. One of the clinical objectives of the study is to demonstrate that 99mTc-Oncardia can be effectively used where the patient does not need to be either physically or pharmacologically stressed for the study.
99mTc-Oncardia is for use with SPECT camera systems. CellPoint also is planning to clinically study 68Ga-Oncardia for use with PET camera systems.
For more information: www.cellpointweb.com


 November 12, 2025
November 12, 2025 









