A novel sinus node ablation technique provides a safe and effective treatment option for patients suffering from symptomatic, drug-resistant inappropriate sinus tachycardia (IST). Results of the Susruta-IST Registry were presented as a late-breaking clinical trial at Heart Rhythm 2021.
August 3, 2021 – A recent study unveiled a novel sinus node ablation technique that provides a safe and effective treatment option for patients suffering from symptomatic, drug-resistant inappropriate sinus tachycardia (IST). Results of the Susruta-IST Registry were presented as a late-breaking clinical trial at Heart Rhythm 2021, the annual meeting of the Heart Rhythm Society (HRS).
Inappropriate sinus tachycardia (IST) occurs when an individual’s heart rate explicably exceeds 100 beats per minute while at rest. More common in young women than men, IST causes varying symptoms that can be debilitating, often impacting quality of life.[1] Because the causes of IST are unknown, it is sometimes misdiagnosed as a pathological sinus tachycardia, emotional problem and/or mental illness, like depression.[2] Current medical therapies for IST management in drug-refractory or intolerant patients provide suboptimal relief and the current interventional therapy, radio-frequency sinus node (RF-SN) ablation, can have low success rates and high risk of complications.
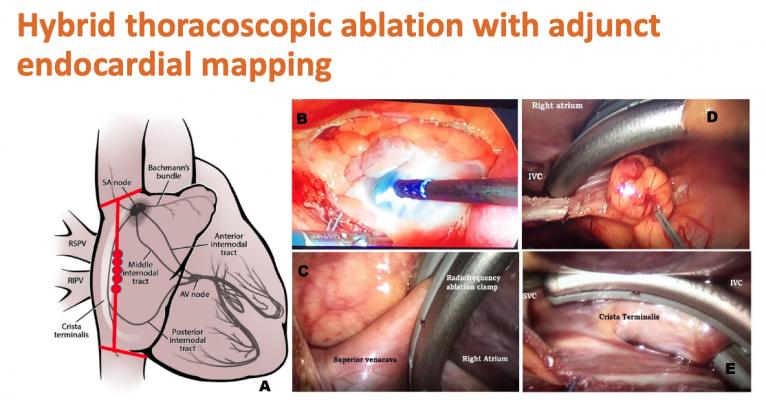
The multicenter, prospective registry compared a novel SN sparing hybrid ablation technique versus conventional RF-SN ablation. The hybrid procedure used video assisted thoracoscopy with an RF bipolar clamp to isolate the superior vena cava, inferior vena cava and the crista terminalis. RF-SN modification was performed by endocardial or epicardial mapping and ablation in the lower half of the SN, which is where it is believed that these rapid electrical
pulses originate.
Of the 100 enrolled patients (hybrid: 50; RF-SN: 50), the mean age was 22.8 years and 82% were women. Outcomes were tracked using implantable cardiac monitors to capture average daily heart rates and peak heart rate following a 6-minute walk test and assessed at baseline, three, six and 12 months. The psychosocial impacts were assessed using the Zung’s SelfRating Depression Scale, Self-Rating Anxiety Scale and Short-Form 36 quality of life questionnaire.
“While there are still some unknowns around inappropriate sinus tachycardia, we do know how unbelievably challenging it can be to live with this heart condition. Often, we see this condition is under-diagnosed or misdiagnosed, especially in women, and for many, the symptoms are debilitating and can greatly alter daily life,” said Dhanunjaya R. Lakkireddy, M.D., FHRS, Executive Medical Director of the Kansas City Heart Rhythm Institute and lead author of the Susruta-IST study. “Our study helps establish a new treatment approach to help get their symptoms under control without major complications. The novel hybrid ablation method is not only a safe and effective treatment option, but it also demonstrates a lower risk of destroying the sinus node, decreases the need for a pacemaker and minimizes the chances of follow-up procedures.”
Normal sinus rhythm and rate was restored in all patients in the hybrid group compared to 84% in the RF-SN group. The hybrid patient group underwent a total of 54 procedures, whereas the RFSN group had a total of 124 procedures with all of them requiring two or more procedures. In addition, the average anxiety score among the hybrid group after six months improved 9 points (±3), compared to only 5 points (±2) in the RF-SN group.
Authors of this study would like to see a randomized trial conducted to help analyze drug treatment compared to the hybrid ablation as treatment options for patients with IST.

Related EP Content:
Find additional HRS 2021 late breaking trials
Find more HRS 2021 conference news
References:


 January 22, 2026
January 22, 2026 









