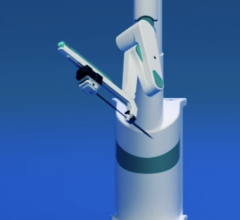
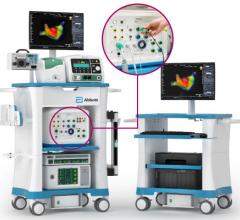
October 13, 2010 – Human trials commenced using a catheter guidance control and imaging (CGCI) system on patients with arrhythmias.
Both studies are being conducted at General Universitario La Paz in Madrid. The initial study will consist of 20 patients in which a highly detailed map of the heart will be created using the Magnetecs Corp. CGCI system’s magnetically guided catheter. The mapping study is expected to be completed by the end of 2010. It will be followed by a study of 40 patients in which mapping and ablation procedures will be conducted using the CGCI system. The second study is expected to be completed in the second quarter of 2011.
CGCI uses eight electromagnets to guide a magnetically tipped catheter, enabling a physician to precisely and consistently control surgical tools in highly dynamic or previously inaccessible environments while enhancing both the physician’s dexterity and the patient’s safety.
Magnetecs expects this study to lead to a CE mark application for commercialization in Europe planned for the first half of 2011. Additional human studies for ablation are expected to lead to approval of the CGCI system for therapeutic procedures used to correct heart arrhythmias.
Studies in Europe and the United States will look to receive FDA 510(k) certification. Magnetecs is currently in the initial stages of planning human studies in the United States at Mount Sinai Medical Center in New York City and in the United Kingdom at St. Mary’s Hospital in London.
For more information: www.magnetecs.com


 January 27, 2026
January 27, 2026