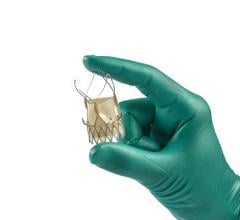
May 7, 2009 - Medtronic Inc. yesterday said it completed enrollment in its investigational device exemption (IDE) study of the Endurant Stent Graft System, which is designed to enable the nonsurgical repair of aortic aneurysms.
Key data from this study, now expected in the second half of 2010, will be used to support the FDA pre-market approval (PMA) submission for U.S. approval of Endurant.
“The clinical community is excited by what this device offers to patients,” said the study’s principal investigator, Dr. Michel Makaroun of the University of Pittsburgh School of Medicine. “The device combines unique improvements with the best features from existing technology. Enthusiastic clinician interest is evident from Endurant’s rapid adoption in Europe, and now accelerated enrollment of the U.S. study.”
Designed to evaluate the safety and effectiveness of the Endurant Stent Graft System for the endovascular aortic repair (EVAR) of abdominal aneurysms, the U.S. Endurant IDE study was enrolled two months ahead of schedule, having started in 2008 and involving 30 sites.
For more information: www.medtronic.com


 October 23, 2024
October 23, 2024