March 2, 2010 – A robotic cath lab navigation system may potentially raise the standard of care in percutaneous coronary intervention (PCI) by improving standardization, reproducibility and accuracy, according to George W. Vetrovec, M.D., of Virginia Commonwealth University Medical Center, when he spoke at CRT 2010 last week.
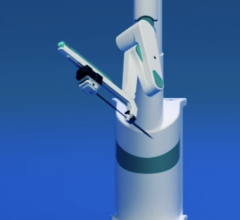
Corindus Vascular Robotics created a vascular robotic system designed to improve patient outcomes while protecting physicians from occupational hazards. Its CorPath vascular robotic system was the subject of a podium presentation last week at CRT.
“The CorPath vascular robotic system can potentially foster a new standard of care for precise stent deployment — which is a big challenge today due to the limited precision associated with manually controlled PCI procedures,” said Dr. Vetrovec, director of the adult cardiac catheterization laboratory, associate chairman of medicine for clinical affairs in the department of internal medicine, and a member of the VCU Health System Board of Directors. “My personal experience using the CorPath system leads me to believe that the system potentially offers interventional cardiologists and their patients several significant procedural advantages over the current manual PCI procedure.”
The CorPath system is designed to significantly reduce radiation exposure, physician fatigue and other occupational hazards to physicians by allowing him or her to operate in an ergonomically correct position while being shielded from harmful and repeated radiation exposure. Many doctors also face chronic orthopedic ailments and fatigue due to the required use of heavy lead protection garments.
Corindus designs, manufactures and commercializes precision vascular robotic systems for use in minimally invasive procedures. The company’s initial product, the CorPath system, is the world’s first to precisely drive coronary guide wires and stent/balloon catheters during PCI procedures performed in a cath lab. While Corindus is focused initially on PCIs, its open-platform technology and IP allow the Company to address other segments of the vascular market — including peripheral and other complex cardiac interventions such as structural heart disease repair.
The Corindus CorPath system is an investigational device in the United States and is limited by federal law to investigational use only.
For more information: www.corindus.com


 January 27, 2026
January 27, 2026