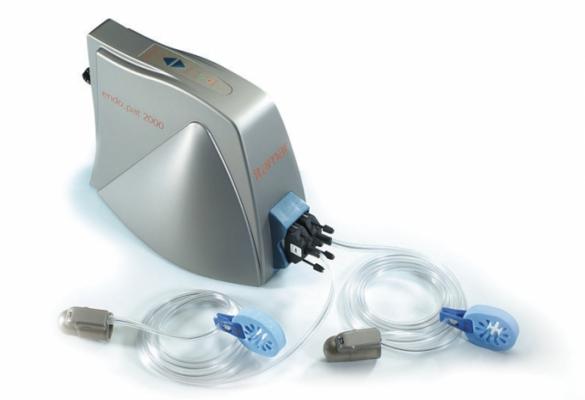
July 18, 2013 — Itamar-Medical announced that the American Medical Association (AMA) has awarded a Current Procedure Terminology (CPT) Category III reimbursement code to a test performed by its EndoPAT proprietary device.
- Reimbursement policy is established by different insurers taking into account both clinical evidence and financial implications. With more than 300 peer reviewed publications and an ever increasing number of physicians using EndoPAT in their clinical practice, it is now expected to fit well into such health plans.
- U.S. physicians will be able to file reimbursement claims specific to Endothelial function assessment, using the EndoPAT with insurers following the introduction of the new CPT code as of Jan. 1, 2014.
- The test has been approved for symptomatic patients and patients already suffering from cardiovascular diseases.
The EndoPAT device is cleared by the U.S. Food and Drug Administration (FDA) and commercially used worldwide by leading research institutes, pharmaceutical companies and private clinics, especially in the United States and Japan.
For more information: www.itamar-medical.com


 November 12, 2025
November 12, 2025 









