Step 2/3
Related Content
August 14, 2023 — The FDA has announced that Datascope/Maquet/Getinge is recalling the Cardiosave Hybrid and Rescue ...
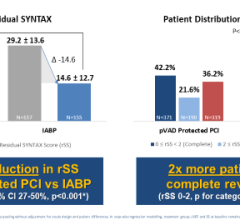
May 19, 2023 — Abiomed, part of Johnson & Johnson MedTech[1], announces results of a third-party analysis showing that ...
March 31, 2023 — According to statement issued by the U.S. Food and Drug Administration (FDA), Datascope, a subsidiary ...
March 20, 2023 — According to a statement issued by the U.S. Food and Drug Administration (FDA), Datascope, a subsidiary ...
January 25, 2023 — According to a new release from the U.S. Food and Drug Administration (FDA), Datascope, a subsidiary ...
The U.S. Food and Drug Administration (FDA) has issued a Class I recall on the Arrow AutoCAT 2 and AC3 Intra-Aortic ...
October 26, 2022 — Abiomed (Nasdaq: ABMD) announces a new program to address healthcare disparities in underserved ...
December 16, 2021 — Datascope/Getinge/Maquet has recalled the Cardiosave Hybrid and Cardiosave Rescue Intra-Aortic ...
November 2, 2021 — Datascope/Getinge/Maquet is recalling its Cardiosave Hybrid/Rescue Intra-Aortic Balloon Pumps (IABP) ...
July 24, 2019 — The U.S. Food and Drug Administration announced Maquet/Datascope is recalling all intra-aortic balloon ...

 August 14, 2023
August 14, 2023