July 16, 2013 — ImaCor Inc. announced the implementation of the 24/7 hTEE Clinical Support Program.
ImaCor’s 24/7 hTEE Clinical Support Program ensures that a senior member of the company’s clinical specialist team is always available via phone, text and video-chat modalities to provide support to clinicians working at the patient bedside. 24/7 hTEE Clinical Support is an enhancement to the ImaCor Customer Service Program, and aligns with ImaCor’s educational commitment to their customers. The launch of this program coincides with the start of the critical care fellowship year.
“While introducing hTEE into our Neurointensive Care Unit, our team benefited from ImaCor's 24/7 hTEE Clinical Support Program,” commented Jacqueline S. Urtecho M.D., Neurointensive Care Physician at Thomas Jefferson University Hospital. “The ability to video-chat with an hTEE clinical specialist at any time complements the team’s ongoing training and ensures that we can apply hTEE confidently whenever our patients need it.”
“Around-the-clock guidance is often necessary during the implementation of new technologies,” said Donald Reiff M.D., director of the Trauma/Burn Unit at the University of Alabama Hospital at Birmingham. “ImaCor’s 24/7 hTEE Clinical Support Program is a best practice in the business, enabling any member of our team — including Fellows and Physician Assistants — to connect with an ImaCor hTEE specialist for day or night support.”
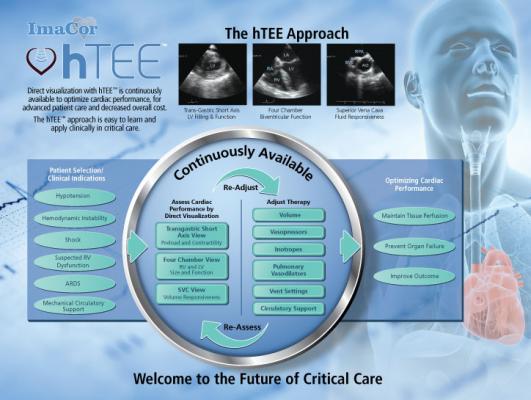
hTEE is the only technology in critical care that provides continuously available, direct visualization of heart filling and function in the ICU, enabling optimal post-operative hemodynamic management.
For more information: www.imacorinc.com


 June 13, 2024
June 13, 2024