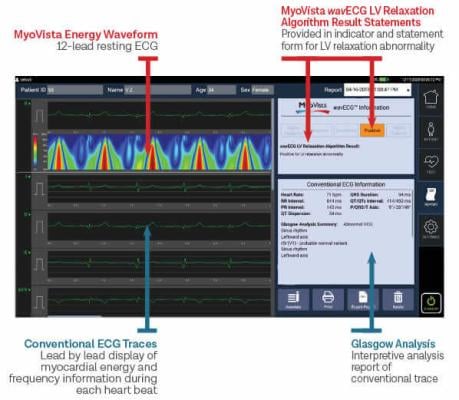
October 19, 2022 — Heart Test Laboratories, Inc. d/b/a HeartSciences, a medical technology company focused on saving lives by making an ECG (also known as an EKG) a far more valuable screening tool through the use of AI, today reports that the American Medical Association (AMA) has issued new industry-first Category III Current Procedural Terminology (CPT) codes for novel AI assistive algorithmic ECG risk assessment for cardiac dysfunction. These codes are expected to cover the AI cardiac dysfunction algorithm incorporated in the Company’s MyoVista Wavelet ECG Cardiac Testing Device currently in development for FDA De Novo submission and clearance. The codes are in addition to the long-established CPT codes in place for the conventional ECG.
CPT Category III codes are designed to facilitate the use, adoption, and potential reimbursement of emerging technologies. The new CPT codes go into effect in the CPT codebook on January 1, 2023.
“This is a major milestone for HeartSciences. The issuance of new CPT codes by the AMA acknowledges the importance of AI analysis of cardiac dysfunction using an ECG. It also further validates the commercial opportunity for the MyoVista,” stated Andrew Simpson, Chief Executive Officer of HeartSciences. “One of the most significant needs in healthcare is the ability to detect cardiac dysfunction early. An AI-enabled ECG is a relatively low-cost, simple, and quick way to do that. Millions of ECG tests are performed every week and we believe adding detection of cardiac dysfunction to the ECG would make it a far more valuable heart screening tool.”
For more information: https://www.heartsciences.com


 September 24, 2025
September 24, 2025