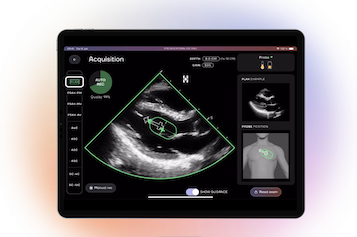
April 15, 2025 – DESKi recently announced FDA clearance for HeartFocus, its AI-enabled heart exam software that empowers any healthcare professional, including novices, to perform clinical-quality heart scans from any compatible device after just a few hours of training.
The FDA also approved DESKi’s Predetermined Change Control Plan (PCCP) for HeartFocus’ growth opportunities. This clears the way for faster collaboration with other ultrasound systems, so the technology can reach more patients sooner.
“Heart disease is the number one killer worldwide, and echocardiography is the first step to receiving a diagnosis and proper treatment. However, there is a critical shortage of cardiologists and expert sonographers to meet demand. HeartFocus bridges that gap, empowering any healthcare provider, anywhere, to capture cardiac images with precision and confidence,” said Bertrand Moal, PhD, MD, CEO of DESKi. “This clearance affirms our technology and accelerates our ability to bring earlier diagnosis and life-saving care to all who need it, including rural and underserved communities.”
The efficacy of HeartFocus was validated in a landmark international clinical trial conducted in partnership with Northwell Health in the United States and the University Hospital of Bordeaux (CHU) in France. In a blind review, 100% of novice-conducted scans using HeartFocus met diagnostic standards for all primary endpoints and were comparable to those completed by expert sonographers.
“The clinical trial results and subsequent FDA clearance mark a major step forward in making echocardiography more accessible in the community,” said Dr. Varinder P. Singh, SVP, Global and International Health, Northwell. “Proving that it can successfully guide any healthcare professional to capture high-quality heart scans, HeartFocus will help patients get diagnosed sooner and move more quickly toward the treatment they need.”
“HeartFocus is opening the door to a new era in cardiac imaging,” said Stéphane Lafitte, Professor of Cardiology at CHU de Bordeaux. “We’ve proven that AI can empower novices to reliably perform echocardiograms, a breakthrough that could reshape how we diagnose and treat heart disease. It’s an exciting advancement for the field of cardiology, with the potential to extend high-quality care to more patients globally than ever before.”
For more information, visit www.heartfocus.ai


 January 15, 2026
January 15, 2026 









