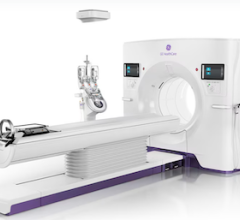
June 9, 2008 - Siemens Medical Solutions gained FDA 510(k) market clearance for the SOMATOM Definition AS, what is said to be the first world's first adaptive CT scanner, adapting to each patient's clinical situation, available in 40-slice, 64-slice and 128-slice configurations.
The new system reportedly provides a benefit with the Adaptive Dose Shield technology, eliminating unnecessary overradiation. The Adaptive 4D Spiral mode of the SOMATOM Definition AS is able to address functional imaging (perfusion images of blood flow over time) of whole organs. This allows Siemens to offer dynamic information of up to 27 centimeters. In the case of a stroke, physicians can use whole-organ perfusion imaging not only for a small part of the brain, but for all of it.
The SOMATOM Definition AS comes in multiple configurations, each tailored to a hospital's workflow and clinical needs, with a goal to make the most complex procedures routine. The technology couples multiple components in a dynamic manner: a large-volume coverage area with a 200-cm scan range and up to 300 msec rotation time, 78-cm gantry bore, and the ability to add a high-capacity 660-pound patient table.
These features reportedly allow even the most clinically challenging patients (i.e., trauma patients) to be imaged rapidly, from head to toe. The system can be upgraded to other configurations with minimal downtime. This allows the technology to grow with the institution's needs while minimizing downtime and loss of service, according to the company.
The SOMATOM Definition AS is also able to adapt to the space constraints many facilities face today. According to Siemens, it requires very little floor space, with an 18-m2 footprint. This allows the Definition AS to fit into rooms that have traditionally been too small for high-end CT scanners.
The system comes in multiple configurations including the Somatom Definition - AS+ Fast Care/AS+ Excel CT, Somatom Definition - AS 64 FAST CARE/64 Excel CT and the Somatom Definition - AS 20/40 CT.
For more information: www.usa.siemens.com/medical


 February 02, 2026
February 02, 2026