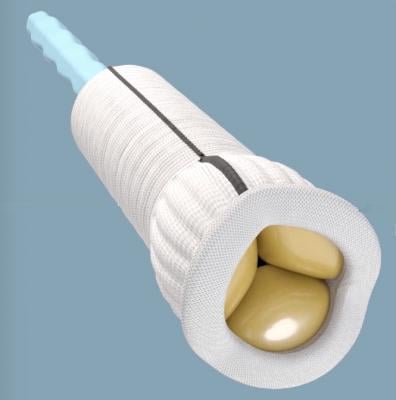
July 15, 2020 — The U.S. Food and Drug Administration (FDA) granted clearance for the Edwards Lifesciences Corp. Konect Resilia aortic valved conduit (AVC), the first ready-to-implant solution for bio-Bentall procedures, a complex surgery that involves replacement of a patient's aortic valve, aortic root and the ascending aorta.
The Konect device is the company's latest innovation offering the advanced Resilia tissue, which incorporates integrity-preservation technology that may help improve valve durability. The Resilia tissue technology also allows devices to be stored under dry packaging conditions, facilitating ease of use during patient care.
"Until now, surgeons did not have a pre-assembled option that was FDA approved, so those utilizing bovine tissue valves for Bentall procedures needed to manually assemble a valve with a conduit in the operating room," said Joseph E. Bavaria, M.D., Brooke Roberts-William M. Measey professor of surgery and vice chief of the division of cardiovascular surgery, University of Pennsylvania. "The Konect device represents a meaningful advancement that offers surgeons a preassembled device with two leading technologies, which can streamline treatment for patients requiring this complex and technical procedure."
Up to 30% of Bentall procedures are performed in an emergency setting. These procedures are performed when patients with valve disease experience a combination of issues with the aorta, including:
• Aneurysm, a bulging in the aorta that can be life-threatening if it ruptures. Bicuspid aortic valve disease is the leading cause of aortic aneurysms.
• Regurgitation, the leakage of blood backwards into the heart due to the leaflets of the valve not closing properly.
• Separation or tears in the walls of the aorta.
• Marfan's syndrome, a birth defect in connective tissue that weakens the aortic wall.
"The Konect device combines Edwards' expertise innovating bovine pericardial tissue technologies, such as Resilia, with the proven clinical history of the Gelweave Valsalva graft," said Daveen Chopra, Edwards' corporate vice president, surgical structural heart. "Because the typical patient is under the age of 60, advanced technologies such as the Konect device with the Resilia tissue might provide extended valve durability for a more active patient population."
Edwards is dedicated to partnering with clinicians to develop patient-centric innovations for complex surgical structural heart procedures that improve long-term care and outcomes for patients. For patients with mitral valve disease in which there is no standard solution for treatment, the recently launched Physio Flex annuloplasty ring is the first-ever product of its kind that mimics the natural anatomy of the mitral valve. Edwards is also investing in innovations in the field of beating heart mitral valve therapies, more durable surgical mitral tissue valve replacement, minimally invasive surgical suture securing systems and other surgical structural heart devices.
Dr. Bavaria is a consultant to Edwards Lifesciences.
For morte information: www.edwards.com


 January 15, 2026
January 15, 2026