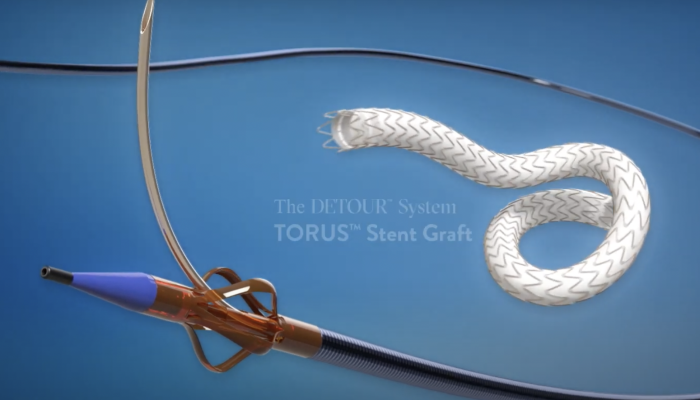
Percutaneous Transmural Arterial Bypass (PTAB) using the DETOUR System (Graphic: Business Wire)
June 16, 2023 — Endologix LLC, a privately held, global medical device company, dedicated to providing disruptive therapies for the interventional treatment of vascular disease, today announced the 24-month results of the DETOUR2 Study. Percutaneous Transmural Arterial Bypass (PTAB) with the DETOUR System, recently received PMA Approval from the FDA on June 7, 2023. This system offers a unique approach to treating complex peripheral arterial disease (PAD), enabling physicians to percutaneously bypass lesions in the superficial femoral artery, by using stents routed through the femoral vein to restore blood flow to the leg. The DETOUR System is comprised of the ENDOCROSS device and TORUS stent grafts.
The DETOUR2 Study enrolled 202 patients in the United States and Europe. The 24-month results from the study were presented at 2023 Annual Meeting of the Society of Vascular Surgery by one of the study’s principal investigators, Dr. Sean Lyden, Chairman of the Department of Vascular Surgery at Cleveland Clinic’s Sydell and Arnold Miller Family Heart, Vascular & Thoracic Institute.
The results* presented included:
- Ninety-six percent (96%) of enrolled patients had chronic total occlusions, with a mean lesion length of 32.7cm.
- Technical success was achieved in 100% of treated patients and the primary safety endpoint was surpassed with a 30-day MAE rate of 7.0%
- The freedom from CD-TLR at 24 months was 76.7%, and secondary patency was 82.3%.
- The freedom from symptomatic DVT was 96.5% at 24 months.
- The freedom from major lower limb amputation was 98.5% at 24 months.
“The two-year results from the DETOUR2 Study are encouraging and demonstrate PTAB using the DETOUR System offers good patency rates in long SFA lesions. As noted in the conclusion of the presentation, the two-year data mimics those of surgical bypass without the need for general anesthesia, long length of stay, and high risk of complications. We look forward to continuing to study the DETOUR System,” said Dr. Lyden.
“We are delighted to present the two-year results of the DETOUR2 Study which investigates the use of the PTAB therapy in patients with very long SFA lesions” said Professor Matt Thompson, MD, President and CEO of Endologix. “The results suggest that the DETOUR System offers a viable approach in patients where open surgery is the currently recommended treatment. We are excited to see more patients benefit from this unique approach to the treatment of complex PAD.”
*Lyden et al. Durability of Percutaneous Bypass for Treatment of Femoropopliteal Disease: Two-year Outcomes of the DETOUR-2 Study. VAM 2023
For more information: www.endologix.com
Related content:


 November 24, 2025
November 24, 2025 









