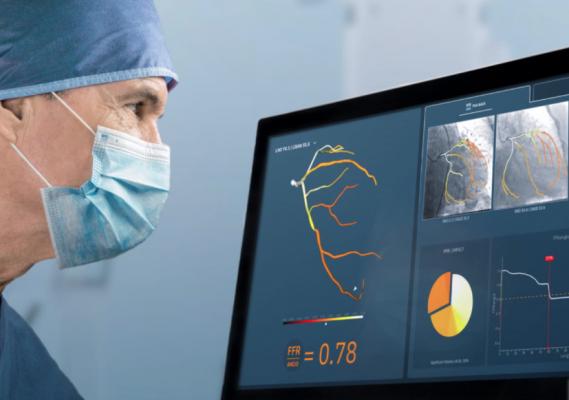
The CathWorks angiography image derived FFR assessment system has received national reimbursement approval in Japan. The system uses a rotational angiography image dataset taken while the patient is in the cath lab to quickly reconstruct a 3-D coronary tree showing FFR numbers for all of the artery segments.
July 26, 2021 – CathWorks announced that the Japanese Ministry of Health, Labour and Welfare (MHLW) has approved the application to provide reimbursement for the CathWorks FFRangio System, making it more broadly available for coronary artery disease decision-making in Japan. The CathWorks FFRangio has already received regulatory approval from the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) in December 2019 and is also commercially available in the United States and Europe.
"The FFRangio System enables us to quickly identify the physiologic significance of coronary artery disease (CAD) without the need for traditional pressure wires or hyperemic agents, which provides significant benefits to clinicians and patients," said Hiroyoshi Yokoi, M.D., President of Fukuoka Sanno Hospital and Vice Chairman, Japanese Association of Cardiovascular Intervention and Therapeutics. "The Japan reimbursement approval is an important step to enable more physicians and patients to have access to this innovative technology."
"When patients present with suspected CAD, it is important that we can quickly and cost-effectively determine the appropriate treatment plan," said Hitoshi Matsuo, M.D., president of Gifu Heart Center. "Our facility has been able to experience firsthand how FFRangio combines artificial intelligence, machine learning, and a streamlined user experience to improve the way we manage and treat our patients."
The FFRangio System is non-invasive and performed intra-procedurally during coronary angiography without adding additional clinical risk or per procedure costs. Clinical trials have demonstrated the FFRangio system is highly accurate compared to traditional wire based fractional flow reserve (FFR) methods. The technology has the potential to positively impact a significant patient population in Japan, where heart disease is the second leading cause of death and coronary artery disease accounts for approximately half of these deaths.[1]
"The reimbursement approval in Japan is a significant and important milestone for CathWorks," said Ramin Mousavi, CEO of CathWorks. "We are pleased with the early adoption of FFRangio in Japan, and grateful for the tremendous physician support of the technology. We look forward to expanding our commercial presence to make FFRangio and non-invasive CAD decision-making available to more clinicians and patients in Japan and around the world."
CathWorks is a medical technology company focused on applying its advanced computational science platform to optimize coronary artery disease (CAD) therapy decisions and elevate coronary angiography from visual assessment to an objective FFR-based decision-making tool for physicians. FFR-guided PCI decision-making is proven to provide significant clinical benefits for patients with coronary artery disease and economic benefits for patients and payers. The company's focus today is specifically on bringing the CathWorks FFRangio System to market to provide quick, precise, and objective intraprocedural wire-free FFR guidance that is practical for every case.
For more information: www.cath.works
Related FFR-angio Content:
VIDEO: Angiography Image-based FFR May Eliminate Need for Pressure Wires — Interview with William Fearon, M.D.
Novel FFR Methods Can Reduce Procedure Time and Cost
CathWorks FFRangio System Receives U.S. FDA Clearance
VIDEO: Example of the Medis QFR Angiography Image Derived FFR System
Medis QAngio XA 3D Receives FDA 510k Clearance
Image-based FFR May Replace Pressure Wires and Adenosine
CathWorks Receives CPT Code for Non-invasive FFRangio Measurements During PCI
Find more news and new technology in the FFR Technology Channel
Reference:
1. Iso H. Changes in Coronary Heart Disease Risk Among Japanese. Circulation. 2008;118:2725-2729.


 November 12, 2025
November 12, 2025 









