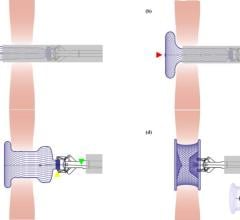
August 12, 2010 – A 52-year-old male heart failure patient in Krakow, Poland, became the first human to receive the PliCath HF System for an epicardial catheter-based ventricular reconstruction (ECVR). The device is designed to treat heart failure (HF) by reducing the enlarged left ventricle (LV) through an anchoring system that is delivered across the cardiac chambers.
By pulling nonadjacent walls of the heart into contact, intervening scar tissue that resulted from a previous heart attack is excluded. It has been known for decades that enlarged hearts are not optimal and the reduction in the size of the LV allows the heart to maintain adequate cardiac output without working so hard.
Lead surgeon, professor Jerzy Sadowski of the Pope John Paul II Hospital, Jagiellonian University in Krakow, expressed great optimism about the device. “The BioVentrix PliCath HF device performed as it was designed. This technology allowed us to perform major reconstruction of the LV on a beating heart in a matter of minutes without incisions. The patient is recovering well. This device has great promise and should help improve the quality of life of our patients suffering from heart failure.”
The patient’s heart size was decreased by 40 percent, while the ejection fraction (EF), the percentage of blood volume ejected from the ventricle after contraction, increased by 15 percent. In extensive animal studies, the PliCath HF System improved the EF by 13 percent; the end diastolic volume by 18 percent; and the end systolic volume (the fully contracted part of the cardiac cycle) by 35 percent. Based on the outcome of this first procedure, it appears that similar results can be achieved in humans. Improvements of this magnitude in the heart size are expected to have a significant clinical effect, since there would be a decrease in the stretching of the remaining heart muscle, allowing it to pump with reduced strain.
“Heart failure, not unlike obesity, has become a problem of epidemic proportions. The reduction in heart size that this device can achieve is the cardiac equivalent of a 40 percent weight loss in a morbidly overweight population,” said Ken Miller, president and CEO of BioVentrix. “In the U.S., nearly 70 percent of the 5 million heart failure patients develop the disease because of a scar on their ventricle from a previous heart attack. This minimally-invasive device will extend effective treatment to a large group of patients where none is currently available.”
The PliCath HF System is an investigational device and is limited by United States law to investigational use.
For more information: www.bioventrix.com


 June 20, 2024
June 20, 2024