July 20, 2007 – Oraganogenesis Inc. announced that it has received regulatory approval in Canada and Europe for BioSTAR, a device that incorporates a bioresorbable collagen scaffold used to treat patent foramen ovale (PFO), a hole in the heart that is usually asymptomatic.
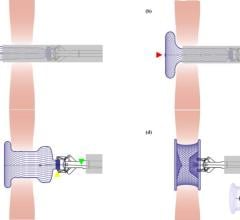
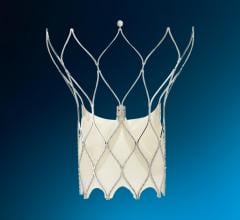
BioSTAR contains FortaFlex, an innovative bioresorbable collagen scaffold that consists of highly purified and very strong sheets of collagen. FortaFlex is a modular platform technology that can be custom bioengineered to meet the needs of a variety of surgical applications using a unique lamination and crosslinking process. The BioSTAR device incorporates the FortaFlex bioresorbable collagen scaffold to function like a pair of small umbrellas. When the BioSTAR device is deployed at the PFO, an umbrella of FortaFlex sits on both the right and left atrium of the heart, effectively closing the hole between the heart chambers. Reportedly, 90-95 percent of the implant is absorbed over time and replaced with the patient’s own tissue. Micro-emboli and unfiltered blood are therefore blocked from passing into the arterial circulation and thus averting a potential stroke.
For more information: www.organogenesis.com


 June 20, 2024
June 20, 2024