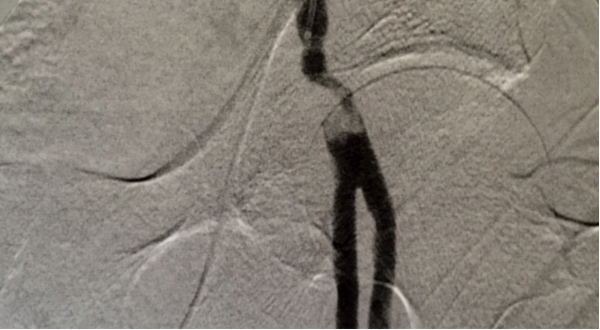
Clot in femoral artery caused by atrial fibrillation
June 11, 2015 - C. Dorn Smith, M.D., vascular surgeon in Kingstree, South Carolina, was successful in using a new device to remove blood clots from a patient with a cold leg using the Aspire mechanical thrombectomy device.
The patient presented with a cold leg and in atrial fibrillation, and Smith performed an angiogram. The angiogram revealed a 30 mm occlusion in the common femoral artery. Patients with atrial fibrillation often produce a clot from the heart that then travels to other areas of the body. Smith made four passes with the Aspire mechanical thrombectomy device. The Aspire device removed the clot, which was evident on a completion angiogram.
"Patients presenting with a cold leg need treatment immediately," said Smith. "The Aspire device was simple to set up and effective in removing the clot. This is a valuable tool in our toolbox to treat cardiovascular disease."
The Aspire mechanical thrombectomy system allows clinicians to instantly start, stop, increase, decrease, pulse or maintain thrombectomy force during a procedure. Aspire mechanical aspirators also aspirate up to 280ml, almost 10 times more than basic syringe-based systems, without multiple messy and time-consuming catheter connections, disconnections, and re-connections to improve speed and performance.
The patented system is available by itself or in kits that include an over-the-wire or rapid exchange thrombectomy catheter. Aspire mechanical aspirators may also be connected to any thrombectomy catheter the clinician chooses.
For more information: www.controlmedtech.com




 January 19, 2026
January 19, 2026 









