January 3, 2011 – Arrow International, a division of Teleflex Medical, issued a class I recall, advising customers to immediately discontinue use of its Ultra 8 French intra-aortic balloon pump (IABP) catheters. The company said these catheters can become stuck in the sheath and the user might not be able to move the catheter forward or backward, causing delay in therapy, bleeding or arterial injury.
The initial recall was initiated in October for Arrow’s Ultra 8 Fr., 30 cc Arrow IAB (Intra Aortic Balloon); Ultra 8 Fr., 40 cc?Arrow Intra-Aortic Balloon (IAB) Catheter with a Fiber Optic Sensor and a Measurement System, 30 cc?Arrow Intra-Aortic Balloon (IAB) Catheter with a Fiber Optic Sensor and a Measurement System. These were manufactured between January 2009 to October 2010 and distributed between November 2009 to October 2010.
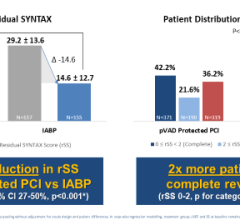
The IABPs are used to treat a variety of cardiac conditions, including heart failure, septic shock and myocardial infarction. They are also used to support and stabilize high-risk patients undergoing diagnostic and nonsurgical procedures.
For more information, contact Arrow customer service at 866.396.2111
For more information: http://service.govdelivery.com/service/view.html?code=USFDA_35

 August 14, 2023
August 14, 2023