July 7, 2022 — Anumana, Inc., an AI-driven health technology company from nference, Inc., today announced the American Medical Association (AMA) has issued new industry-first Category III Current Procedural Terminology (CPT) codes for novel assistive AI algorithmic electrocardiogram risk assessment for cardiac dysfunction, encompassing Anumana’s pipeline of AI-enhanced ECG-based algorithms currently in development for FDA regulatory clearance.
This ground-breaking approval is a major milestone for AI in cardiology, recognizing Anumana’s AI-enhanced ECG-based algorithms as innovative technology necessitating the creation of a new procedure category. CPT Category III codes are designed to facilitate the use, adoption, and potential reimbursement of emerging technologies. The newly approved AMA CPT Category III codes were published on July 1, 2022, and will be effective on January 1, 2023.
“We are delighted that the American Medical Association has approved our application for a new category of CPT codes covering our AI-enhanced ECG algorithm pipeline,” said David McMullin, Chief Business Officer at Anumana. “The new AMA codes mark a significant milestone for Anumana, validating the potential impact of our innovative technology and establishing a pathway for widespread adoption and reimbursement.”
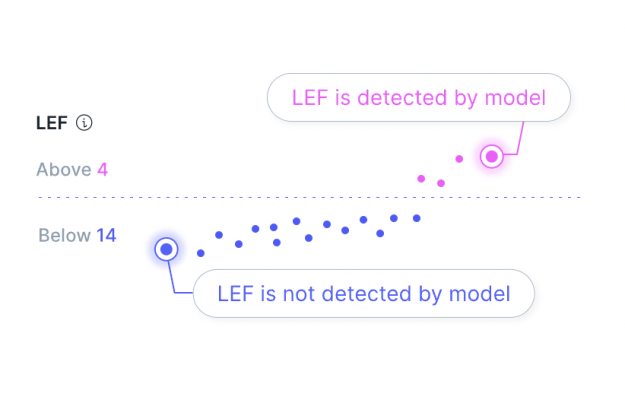
Anumana’s robust pipeline of novel algorithms has been validated by over 50 peer-reviewed publications, and are currently in development as medical devices. Anumana’s pulmonary hypertension algorithm received FDA Breakthrough Device Designation earlier this year, and its low ejection fraction algorithm received FDA Breakthrough Device Designation in 2019 and Emergency Use Authorization for COVID-19 in 2020.
For more information: anumana.ai


 November 12, 2025
November 12, 2025