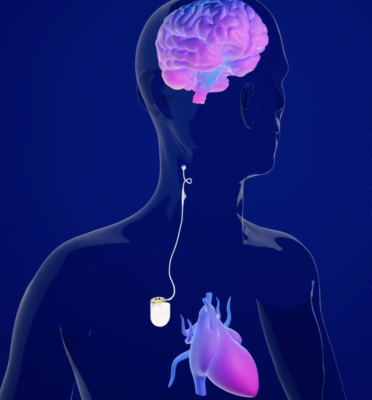
A team at Allina Health Minneapolis Heart Institute at Abbott Northwestern Hospital has successfully completed the first Barostim Baroreflex Activation Therapy implant in the state of Minnesota, according to an announcement from CVRx, Inc. and Allina Health. Barostim is the world’s first FDA-approved neuromodulation heart failure device, which works by stimulating baroreceptors — natural sensors located in the wall of the carotid artery, according to CVRx.Image courtesy: CVRx
February 12, 2023 — A team at Allina Health Minneapolis Heart Institute at Abbott Northwestern Hospital has successfully completed the first Barostim Baroreflex Activation Therapy implant in the state of Minnesota, according to an announcement from CVRx, Inc. and Allina Health.
Barostim is the world’s first FDA-approved neuromodulation heart failure device, which works by stimulating baroreceptors — natural sensors located in the wall of the carotid artery, according to CVRx. These sensors tell the nervous system how to regulate heart, kidney and vascular function, and these effects may reduce the heart’s workload and help it pump more efficiently, improving the heart failure symptoms of patients with systolic heart failure.
The procedure was performed by Jeffrey Jim, MD, FACS, Chair of Vascular and Endovascular Surgery, and referred by Peter Eckman, MD, Heart Failure Section Head, at Allina Health Minneapolis Heart Institute.
“Not only are we proud to be offering Barostim at Abbott Northwestern Hospital, but we are excited to be the first hospital in the state of Minnesota to make this novel therapy available for heart failure patients,” said Dr. Eckman. “With this milestone, more patients now have access to a treatment option to help reduce their heart failure symptoms and improve their quality of life when medications alone are not enough.”
The announcement noted that this procedure also marks the first Barostim implant for CVRx on the home front. The Minneapolis, MN-based company received U.S. Food and Drug Administration (FDA) approval for Barostim in August 2019, and now adds Allina Health’s Abbott Northwestern Hospital to its growing, active total implanting sites throughout the U.S. of 178.
In November, 2022, the company launched its new Barostim NEO2 IPG. At that time, it reported that the second-generation device reduces the size of the IPG by 10% and extends battery life by 20%, reducing the frequency of device replacements for patients and their providers. It further added that the Barostim NEO2 also offers a streamlined design with a single lead port (compared with two in the prior generation device) to further simplify the implant procedure. All Barostim Programmer models are compatible with the new IPG model. According to Nadim Yared, President and CEO of CVRx, in a written statement: "The new Barostim NEO2 is another leap forward in improving the patient and provider experience with Barostim Therapy. These upgrades enable improved longevity using a smaller footprint and allow physicians to implant the device more easily than ever before.”
Patient Experience
The patient who received the Barostim implant is 75-year-old James Bennett of Brainerd, Minnesota. The father of three and grandfather of eight, he has had three heart attacks in the last three years, which led to congestive heart failure.
“I felt weak and tired all the time and knew I didn’t want to spend my life sitting in the living room chair. When my doctor recommended Barostim and gave me the option to feel better, I was ready for a change,” said Bennett. Following the implant and subsequent clinical follow-ups with his physician, Bennett has begun to feel symptom improvements from the therapy, reported the company in its announcement. “My wife and I have 3.5 acres of land and, since receiving Barostim, I’m back mowing. I’m able to do yardwork, get outside and spend time being active,” said Bennett.
"Congratulations to Dr. Jim, Dr. Eckman and Abbott Northwestern Hospital on successfully completing this first implant procedure of Barostim," said Nadim Yared, President and CEO of CVRx. "We value our collaboration with them and commend their ongoing efforts to advance the treatment of heart failure symptoms.”
CVRx is a commercial-stage medical device company focused on the developing, manufacturing and commercializing innovative neuromodulation solutions for patients with cardiovascular diseases. The therapy is designed to restore balance to the autonomic nervous system and thereby reduce the symptoms of heart failure. Barostim received the FDA Breakthrough Device designation and is FDA-approved for use in heart failure patients in the U.S. It has also received the CE Mark for heart failure and resistant hypertension in the European Economic Area.
A nonprofit health care system, Allina Health serves patients throughout Minnesota and western Wisconsin through its 90+ clinics, 12 hospital campuses, 14 retail pharmacies, and many specialty care centers and specialty medical services, home care, and emergency medical transportation services.
More information: cvrx.com
Related content:
CVRX REPORTS PRELIMINARY RESULTS OF THE BEAT-HF POST-MARKET RANDOMIZED CLINICAL TRIAL
CVRX RECEIVES MR-CONDITIONAL LABELING APPROVAL FOR ITS BAROSTIM HEART FAILURE SYSTEM


 February 03, 2026
February 03, 2026 









