August 18, 2023 — The U.S. Food and Drug Administration (FDA) announced that Abiomed is recalling the labeling for Impella RP Flex with Smart Assist System Catheter because the catheters’ Instructions for Use (IFU) do not appropriately address precautions for health care providers to take when treating patients whose anticoagulation clotting time is below the recommended value. Patients with central venous lines and cardiac cannulas with systemic anticoagulation below IFU recommendation of 160-180 seconds are most at risk.
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
Please be aware, this recall is a voluntary correction, not a product removal.
Recalled Product
- Product Names: Impella RP Flex with SmartAssist
- Product Codes: See Recall Database Entry
- Model Number: 1000323
- Distribution Dates: November 1, 2022 to present
- Devices Recalled in the U.S.: 65
- Date Initiated by Firm: June 29, 2023
Device Use
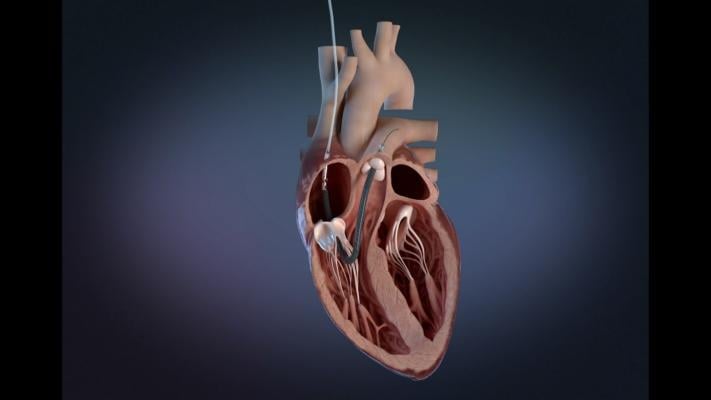
The Impella RP Flex with Smart Assist System Catheter is used for up to 14 days in patients that develop acute right heart failure after left ventricular assist device implantation. The device is placed via the internal jugular vein and supports the right chamber of the heart (ventricle) by pumping blood into the pulmonary artery.
Reason for Recall
Abiomed is recalling the labeling for Impella RP Flex with Smart Assist System Catheter because the catheters' Instructions for Use (IFU) do not appropriately address precautions for health care providers to take when treating patients whose anticoagulation clotting time is below the recommended value. Patients with central venous lines and cardiac cannulas with systemic anticoagulation below IFU recommendation of 160-180 seconds are most at risk.
Please note that clinicians may continue to use the devices. The use of affected catheters may cause serious adverse health consequences including the risk of blood clots or particle deposits forming or death.
There have been 12 reported injuries. There have been no reports of death.
Who May be Affected
- People who receive circulatory support from Impella RP Flex with SmartAssist Catheters
- Health care personnel providing care for people who receive support using the Impella RP Flex with SmartAssist Catheters
What to Do
On June 29, 2023, Abiomed sent all affected customers an Important Medical Device Advisory letter.
To minimize risk of thrombus formation or deposition, the following is recommended:
- Maintain systemic anticoagulation (ACTs of 160-180 seconds) when indwelling central venous lines (i.e., hemodialysis, PA catheters) are present, for the duration of Impella® RP Flex with SmartAssist support as clinically feasible.
- Assess the risk for extraluminal thrombus on indwelling lines (i.e., hemodialysis catheters, PA catheters) placed prior to initiation of support.
- Refer to the recommendations included in the "best practices pathway" on Figure 5.2 of IFU for optimal patient selection. In particular, elements that relate to the risk factors identified above:
- Evidence of end-organ failure (bilirubin >5 or creatinine >4 within 24 hours of implant)
- Active infection (two of the following three: White Blood Cell (WBC) count >12,500, positive blood culture or fever)
- Documented Deep Vein Thrombosis (DVT)
- Patients on right-sided support or Extracorporeal Membrane Oxygenation (ECMO)
Abiomed is revising the IFUs for the Impella® RP Flex with SmartAssist to clarify the risk factors and recommendations related to the potential of thrombus formation or deposition.
Contact Information
Customers with questions about this recall should contact Abiomed's Clinical Support Center at 1-800-422-8666.
Additional Resources
Medical Device Recalls database
How do I report a problem?
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program using an online form, regular mail, or FAX.
Related content:
Impella RP Flex with SmartAssist Receives FDA Approval to Treat Right Heart Failure
Henry Ford Health Cardiologists First in Michigan to Use Heart Recovery Technology
Abiomed at CRT 2023: Benefits of Impella-Supported High Risk PCI and Impella Innovation


 February 03, 2026
February 03, 2026 









