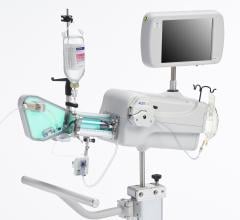
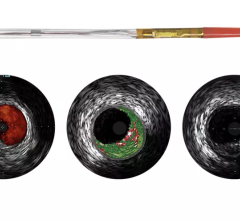
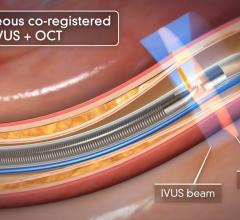
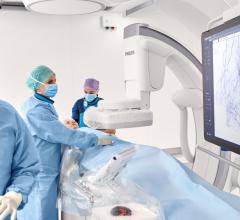
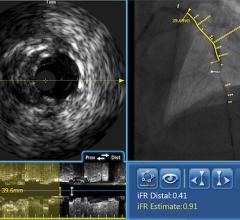
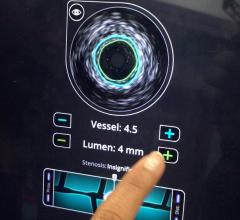
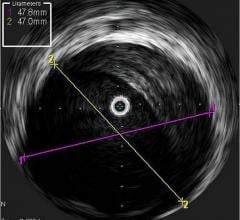
GE Healthcare and Volcano Corp. have announced FDA 510(k) clearance for the integration of Volcano’s intravascular ultrasound imaging (IVUS) capabilities onto the best-of-class Innova all-digital X-ray cath lab imaging system
The digital cardiovascular imaging system is designed to give interventional cardiologists and interventional radiologists a clearer view of coronary and peripheral vessel morphology in a more accessible manner than previously available. Innova IVUS, the product’s commercialized name, is the culmination of an agreement the two companies forged in March.
Comprised of Volcano’s latest PC-based IVUS platform that reduces the size, weight and noise of the IVUS console, the unit can be located in the control room or in other areas outside of the daily traffic pattern of the cath lab.
For more information, contact Kristin Binns, Public Relations Manager, GE Healthcare, at 262-544-3616; or Scott Huennekens, president and CEO and John Dahldorf, CFO, Volcano Corp., at 916-638-8008.


 September 18, 2025
September 18, 2025