May 31, 2019 — Terumo Medical Corp. is recalling the SoloPath Balloon Expandable TransFemoral System and Re-Collapsible ...
Vascular Access
This channel includes news and new technology innovations for vascular access, both venous and arterial access, for percutaneous, transcatheter, interventional devices. In cath lab procedures, an introducer sheath needs to be inserted into the vessel to allow access of guidewires, catheters and devices into the body. Introducer sheaths are self-sealing to serve a stop cock to prevent blood from leaving the body during procedures. There are various access sites that can be accessed, most commonly femoral or radial access, but also the ulnar, axillary and tibiopedal vasciular access routes There are several complications that can occur at the access site and technologies have been developed to reduce these issues, especially with the most common bleeding complications.
May 17, 2019 — At the 40th annual Heart Rhythm Scientific Sessions, May 8-11 in San Francisco, Acutus Medical announced ...
May 15, 2019 — Cordis, a Cardinal Health company, recently announced the full U.S. launch of its Radial 360 portfolio ...

Interventional cardiology has witnessed a rapid and constant evolution in both techniques and device technology since ...
Mladen I. Vidovich, M.D., FACC, FSCAI, chief, section of cardiology, Jesse Brown VA Medical Center, Chicago, and ...
October 4, 2017 — TVA Medical Inc. announced that its everlinQ 4 endoAVF System has received CE Mark in the European ...
This video case study, provided by Gore Medical, is titled "Tackling Complex Cases in Dialysis Access," by John Ross, M ...
March 31, 2017 — The U.S. Food and Drug Administration (FDA) announced it has initiated a Class I recall of the Merit ...
January 31, 2017 — Bard Peripheral Vascular Inc. is recalling the Halo One Thin-Walled Guiding Sheath because the sheath ...
January 10, 2017 — Teleflex Inc. announced that its Arrow VPS Rhythm Device with optional TipTracker technology has been ...
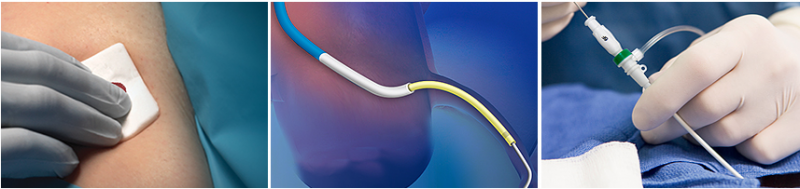
December 7, 2016 — Teleflex Inc. and Vascular Solutions Inc. announced that the companies have entered into a definitive ...
September 20, 2016 — Teleflex Inc. recently displayed its vascular access technologies and a new educational platform ...
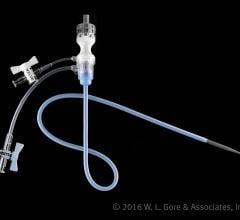
August 30, 2016 — W. L. Gore & Associates Inc. (Gore) announced the commercial availability of the Gore DrySeal Flex ...
August 10, 2016 — Access Scientific introduced the PowerWand XL, a companion to the PowerWand All-in-One, in July. The ...
January 4, 2016 — In a comparison of bleeding complications and mortality between transradial and transfemoral stent ...


 May 31, 2019
May 31, 2019