November 9, 2021 — Results from the largest randomized trial available comparing different closure device strategies ...
TCT
This channel contains news about the annual Transcatheter Cardiovascular Therapeutics (TCT) conference presented by the Cardiovascular Research Foundation (CRF). It includes coverage from the annual meeting and links CRF news. TCT is the premier conference for the subspecialty of interventional cardiology, including the new subspecialty areas of transcatheter structural heart procedures.
November 9, 2021 — Utilizing a magnetically-controlled capsule endoscopy system, the double-blind, randomized OPT-PEACE ...
November 9, 2021 — Six-month outcomes from the randomized RADIANCE-HTN TRIO Trial comparing endovascular ultrasound ...
The optimal delivery of cardiac care is evolving rapidly. A growing number of patients combined with innovative new ...
November 9, 2021 — The primary results of the Fractional Flow Reserve Versus Angiography for Multivessel Evaluation ...
November 9, 2021 — Results from SUGAR trial, a randomized, controlled, multicenter trial conducted exclusively in ...
November 9, 2021 — New five-year data from the SURTAVI trial found that there was no difference in all-cause mortality ...
November 9, 2021 — An economic analysis of data from PARTNER 3, a randomized trial comparing transcatheter aortic valve ...
November 9, 2021 — Use of a novel technique called the quantitative flow ratio (QFR) to precisely identify and measure ...
November 9 2021 — A new large-scale, real-world analysis of Centers for Medicare and Medicaid Services (CMS) outcomes ...
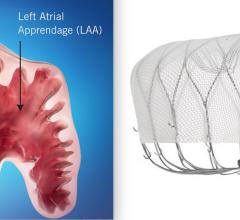
November 8, 2021 – SWISS-APERO is the first randomized clinical trial comparing the Abbott Amulet left atrial appendage ...
November 8, 2021 — Results from a clinical trial of the Edwards Lifesciences Evoque transcatheter tricuspid valve ...
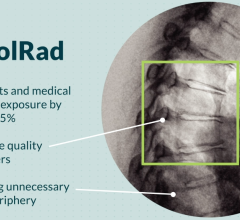
November 5, 2021 – An FDA-approved device used during cardiac cath lab procedures cut radiation exposure for ...
This is an example of the Medis Medical Imaging Quantitative Flow Ratio (QFR) system that offers a fractional flow ...
This is an example of the Siemens Corindus CorPath Cath lab robotic system being used for a percutaneous coronary ...
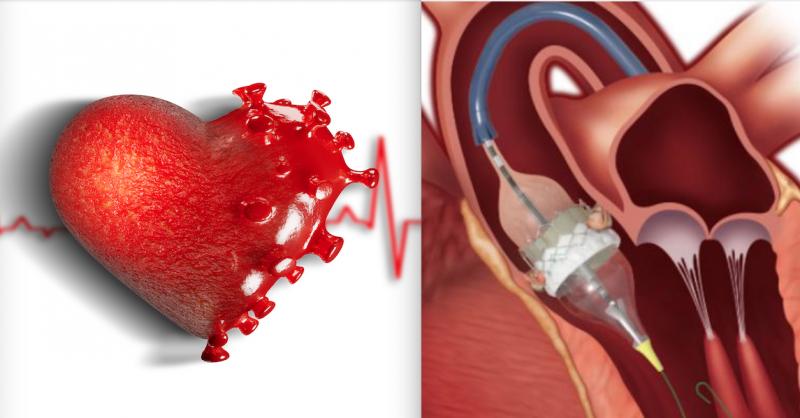
December 1, 2020 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...

 November 09, 2021
November 09, 2021