October 3, 2019 – The three-year results from the COAPT Trial demonstrated that reducing severe secondary mitral ...
TCT
This channel contains news about the annual Transcatheter Cardiovascular Therapeutics (TCT) conference presented by the Cardiovascular Research Foundation (CRF). It includes coverage from the annual meeting and links CRF news. TCT is the premier conference for the subspecialty of interventional cardiology, including the new subspecialty areas of transcatheter structural heart procedures.
October 2, 2019 – The first randomized trial to compare the safety and efficacy of the new Boston Scientific Acurate neo ...
October 2, 2019 – The Abbott Portico FDA investigational device exemption (IDE) study found that 30-day safety and one ...
The optimal delivery of cardiac care is evolving rapidly. A growing number of patients combined with innovative new ...
September 30, 2019 – The first randomized trial to compare a durable polymer drug-eluting stent to a polymer-free drug ...
September 30, 2019 — A biodegradable polymer everolimus-eluting stent (BP-EES) followed by four months of dual ...
September 30, 2019 – Data from the EVOLVE Short DAPT study found that shortened three-month dual antiplatelet therapy ...
September 27, 2019 — Ancora Heart Inc. announced results from an interim analysis of heart failure patients treated in ...
September 27, 2019 — Abiomed’s newest heart pump, the Impella 5.5 with SmartAssist, has received U.S. Food and Drug ...
September 27, 2019 — EchoPixel introduced what it calls the first-ever intraoperative software to provide naked-eye ...
September 26, 2019 — New data from the Phase IV independent TWILIGHT trial showed Brilinta (ticagrelor) monotherapy redu ...
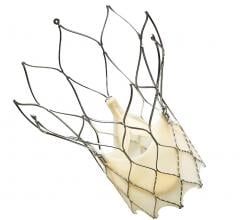
September 24, 2019 – Medtronic announced U.S. Food and Drug Administration (FDA) approval and U.S. launch of the Evolut ...
There was a 77 percent increase in survival in cardiogenic shock patients treated using a new protocol in the National ...
September 20, 2019 — BioTrace Medical Inc. announced the company’s key activities at the 31st annual Transcatheter ...
September 17, 2019 — The U.S. Food and Drug Administration (FDA) has cleared the Artis icono, a high-precision family of ...
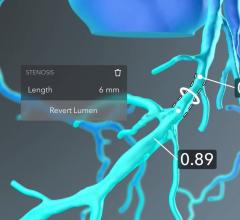
September 12, 2019 — HeartFlow Inc. has obtained clearance from the U.S. Food and Drug Administration (FDA) for the ...


 October 03, 2019
October 03, 2019