February 20, 2012 — Edwards Lifesciences Corp. announced that it received CE mark in Europe for its Edwards Intuity ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
February 16, 2012 — Siemens Healthcare received clearance from the U.S. Food and Drug Administration (FDA) for syngo Aor ...
Being a child of the late 1970s and 1980s, I was a big Star Wars fan. Somewhere in my basement I still have the action ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
February 3, 2012 — Colibri Heart Valve received an notification it received patents for a new transcatheter heart valve ...
February 3, 2012 — Medtronic Inc. issued a statement on the results of two studies evaluating the use of the Medtronic ...

Based on my observations and those of the doctors on the Diagnostic & Interventional Cardiology Editorial Advisory Board ...

With the approval of the Sapien valve in November 2011, transcatheter aortic valve implantation (TAVI) technology is ...
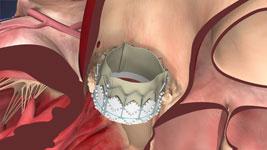
Recently, the U.S. Food and Drug Administration (FDA) has approved the Edwards Sapien transcatheter heart valve for ...
January 30, 2012 — Medtronic, Inc. announced the completion of patient enrollment in the extreme risk study in its ...
January 30, 2012 - The 2012 American College of Cardiology (ACC) meeting in Chicago, March 24-26, includes five sessions ...
January 26, 2012 — Heart Hospital of Austin opened a heart valve clinic, the first of its kind in the region. The clinic ...
January 25, 2012 — Which Medical Device announced the winner of its Device of the Year Award 2011 in the cardiology ...
January 25, 2012 — Terumo Cardiovascular Systems entered into an exclusive agreement with LAAx Inc. to distribute the ...
January 17, 2012 – Terumo Americas Holding Inc., a U.S. subsidiary of Japan's Terumo Corp., announced it acquired Onset ...
January 4, 2012 – ECRI, an independent nonprofit that researches the best approaches to improving patient care, listed ...


 February 20, 2012
February 20, 2012










