October 8, 2009 – A five-judge panel in the German Federal Patent Court in Munich upheld the validity of an AGA ...
Structural Heart Occluders
This channel includes news and new technology innovations about structural heart occluders. These include information of surgical repair and transcatheter closure of PFO, VSD, ASD and LAA. Devices include the Amplatzer and the Gore Helex
September 17, 2009 – NMT Medical Inc. said yesterday it will commence data analysis for its landmark STARFlex ...
August 24, 2009 – Physicians at The Mount Sinai Medical Center were the first in the country to perform a ...
July 31, 2009 – A United Kingdom court has determined Occlutech GmbH’s products do not infringe on AGA Medical ...
May 11, 2009 - Medtronic Inc. today announced the successful implant of its Cardioblate Closure Left Atrial ...
April 10, 2009 - NMT Medical Inc. said this week data analysis for its STARFlex device for patent foramen ovale ...
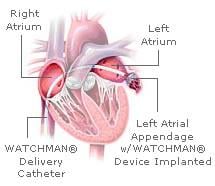
April 2, 2009 - A device implanted in the heart using minimally invasive techniques may replace the most widely ...
March 6, 2009 - Atritech Inc. will release PROTECT AF trial results on WATCHMAN LAA closure technology comparing ...
March 5, 2009 - Reducing the frequency and severity of disabling migraines is crucial for quality of life and a ...
March 4, 2009 - W. L. Gore & Associates has earned a spot on Fast Company magazine’s 2009 “Fast 50” list of the ...
February 5, 2009 - AGA Medical Corp. last week said following its previous favorable structural heart occluder ...
December 22, 2008 - W. L. Gore & Associates last week said Rush University Medical Center in Chicago is the first ...
At TCT 2008 AGA Medical Corp. highlighted its self-expanding AMPLATZER Muscular VSD Occluder for complex ...
August 18, 2008 - Atritech Inc. today said it filed its pre-market approval application (PMA) with the FDA for its ...
W. L. Gore & Associates (Gore) announced that the FDA granted approval for the use of GORE HELEX Septal Occluder ...

 October 07, 2009
October 07, 2009





