May 14, 2007 — More than 25 leading heart advocacy groups have joined to form the Sudden Cardiac Arrest (SCA) Coalition ...
Stents
This channel includes news and new technology innovations for stents, also called vascular scaffolds. Stents are used to help prop open a vessel treated by balloon angioplasty because of the barotrauma caused by the extreme stretching of vessel walls. The stent enables to vessel to heal in an open position with collapsing. Drug eluting stents (DES) are coated in anti-proliferative drugs to precent scar tissue growth which can cause restenosis and occlude the vessel. DES require antiplatelet therapy because the drug carrier polymer on DES can cause thrombus inside the stent, even years after treatment, which is why bare metal stents are still used in some patients. This page includes news on coronary stents, carotid stents, peripheral stents, bioresorbable stents, and renal stents.
May 11, 2007 — Northwestern University in Chicago has published results of a one-year study of roughly 5,500 patients ...
May 9 — Brookwood Pharmaceuticals, Inc. and Targeted Technology Ventures LLC have announced the creation of a ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
The 6F Luminexx 3 Biliary Stent is the benchmark for self-expanding nitinol stent visibility under fluoroscopy ...
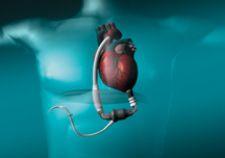
In the simplest of terms, a ventricular assist device (VAD) is a battery-operated mechanical pump that helps a weakened ...
May 8, 2007 - OBS Medical and Physio-Control Inc., a wholly-owned subsidiary of Medtronic Inc., announced they have ...
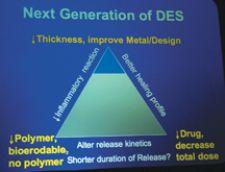
Examples of physicians’ declining use of drug-eluting stents are growing.
According to Dr. Louis Cannon, Cardiac & ...

When it comes to treating patients suffering from heart failure, which is typically defined as a heart whose ...
May 7, 2007 — Citing issues related to potentially suboptimal therapeutic dosing of paclitaxel, Conor Medsystems, LLC ...
April 30, 2007 — Carotid stenting can be performed on any age patient, but should be undertaken with caution and ...
April 9, 2007 — The drug used in Johnson & Johnson’s drug-eluting stent — produced by J&J-owned Cordis — does not ...
April 9, 2007 — MIV Therapeutics Inc. has announced that its recent bench test confirmed that merging Biosync Scientific ...
The results of Boston Scientific's pivotal SPIRIT III clinical trial reaffirmed prior safety and efficacy data ...
Boston Scientific Corp. announced two-year results from the TAXUS V In-Stent Restenosis (ISR) trial, demonstrating ...
The FDA has granted clearance to the Cordis Endovascular division of Cordis Corp. to market its PRECISE RX ...

 May 13, 2007
May 13, 2007


