July 22, 2009 – W. L. Gore & Associates today said it received FDA approval to market its GORE VIABAHN ...
Stents
This channel includes news and new technology innovations for stents, also called vascular scaffolds. Stents are used to help prop open a vessel treated by balloon angioplasty because of the barotrauma caused by the extreme stretching of vessel walls. The stent enables to vessel to heal in an open position with collapsing. Drug eluting stents (DES) are coated in anti-proliferative drugs to precent scar tissue growth which can cause restenosis and occlude the vessel. DES require antiplatelet therapy because the drug carrier polymer on DES can cause thrombus inside the stent, even years after treatment, which is why bare metal stents are still used in some patients. This page includes news on coronary stents, carotid stents, peripheral stents, bioresorbable stents, and renal stents.
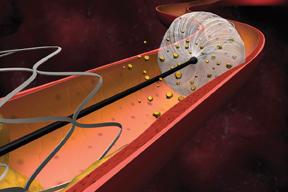
When stenting emerged as a minimally- invasive treatment for clogged arteries associated with peripheral artery ...
July 16, 2009 – Boston Scientific Corp. today said its received approval from the FDA to market its TAXUS Liberte ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
July 14, 2009 – Despite the fact that drug-eluting stent (DES) implantation reduces restenosis and the need for ...
July 13, 2009 – Daiichi Sankyo Inc. and Eli Lilly and Company said Friday the FDA approved Effient (prasugrel) ...
July 9, 2009 – Coherex Medical Inc. today said its Coherex FlatStent EF PFO Closure System has been granted CE ...
June 30, 2009 - Stentys this week said it secured the second tranche of its series B financing, which will enable ...
June 24, 2009 – CorNova Inc. said this week it received CE mark approval for its Valecor Platinum Coronary Stent ...
June 23, 2009 – Abbott said today it received CE mark (Conformite Europeenne) for its next-generation Xience PRIME ...
June 18, 2009 – Abbott this week initiated SPIRIT PRIME, a clinical trial to study the performance of the company ...
June 11, 2009 - Cook Medical announced today the completion of patient enrollment in its REFORM clinical trial ...
June 8, 2009 - The Bypass Angioplasty Revascularization Investigation With Diabetes Trial (BARI-2D) is a useful ...
June 5, 2009 - Raydiance Corp. introduced Smart Light MD — a software-controlled ultrafast laser for manufacturing ...
June 2, 2009 – During their report Friday at the 63rd Annual Meeting of the Society for Vascular Surgery ...
June 1, 2009 - Devax Inc. today said FDA has conditionally approved an investigational device exemption (IDE) for ...


 July 22, 2009
July 22, 2009






