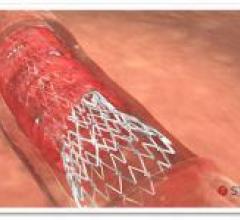
October 15, 2015 — Two new advances in stent technology announced in recent days further reinforce the effectiveness of ...
Stents
This channel includes news and new technology innovations for stents, also called vascular scaffolds. Stents are used to help prop open a vessel treated by balloon angioplasty because of the barotrauma caused by the extreme stretching of vessel walls. The stent enables to vessel to heal in an open position with collapsing. Drug eluting stents (DES) are coated in anti-proliferative drugs to precent scar tissue growth which can cause restenosis and occlude the vessel. DES require antiplatelet therapy because the drug carrier polymer on DES can cause thrombus inside the stent, even years after treatment, which is why bare metal stents are still used in some patients. This page includes news on coronary stents, carotid stents, peripheral stents, bioresorbable stents, and renal stents.
October 15, 2015 — Biotronik has announced results from the BIOSOLVE-II trial, investigating the safety and clinical ...
October 14, 2015 — Tryton Medical Inc. announced results from the pivotal Tryton Confirmatory Study confirming the ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
October 5, 2015 — The U.S. Food and Drug Administration (FDA) has approved Boston Scientific’s Synergy Bioabsorbable ...
October 2, 2015 — Tryton Medical Inc. announced that results of a post hoc analysis of the pivotal Tryton Randomized ...
October 1, 2015 — Kimihiko Kichikawa, M.D., department of radiology at Nara Medical University in Japan, reported two ...
October 1, 2015 — Stentys announced that the company’s drug-eluting stent received CE Mark for treatment of below-the ...
September 28, 2015 — New 12-month clinical trial outcomes assessing the safety and performance of the Boston Scientific ...
September 23, 2015 — CeloNova BioSciences Inc. announced that it has received conditional approval to start an ...

September 4, 2015 — A drug-eluting coronary stent made of absorbable material performed similarly to gold-standard metal ...
September 1, 2015 — A bioresorbable drug-eluting coronary stent showed similar efficacy and safety results compared to a ...
August 28, 2015 — PinnacleHealth CardioVascular Institute enrolled the first patient in Pennsylvania and second in the ...
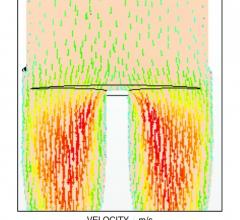
August 27, 2015 — The use of computational fluid dynamics (CFD) modeling has been used for years to better engineer ...
August 26, 2014 — The Cardiovascular Research Foundation (CRF) announced the late-breaking trials and first report ...
August 20, 2015 — Boston Scientific Corp. has received U.S. Food and Drug Administration (FDA) approval for the Innova ...

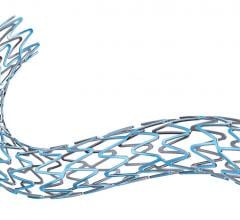
 October 15, 2015
October 15, 2015