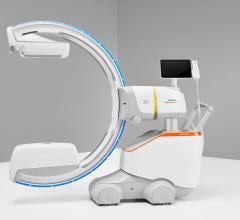
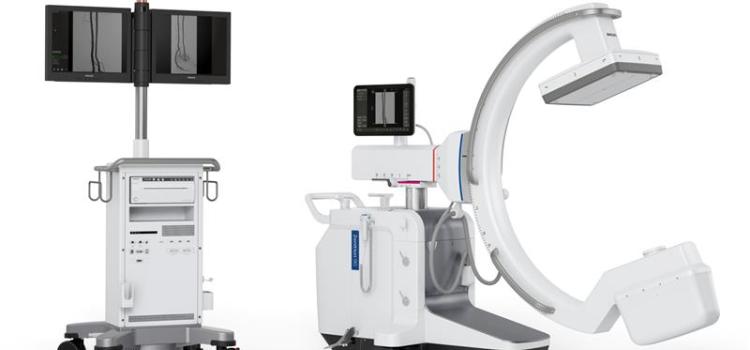
June 19, 2024 —Royal Philips, a global leader in health technology, today announced the launch of Philips Image Guided Therapy Mobile C-arm System 9000 – Zenition 90 Motorized, designed to help ...
Mobile C-Arms
Mobile C-Arms are a type of angiography X-ray system that can be used for a variety of diagnostic imaging and minimally invasive surgeries. This channel includes news and new technology innovations for mobile C-arms and mini C-arms.
June 19, 2024 —Royal Philips, a global leader in health technology, today announced the launch of Philips Image Guided ...
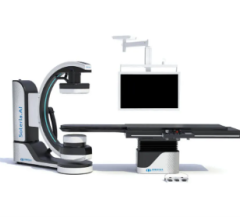
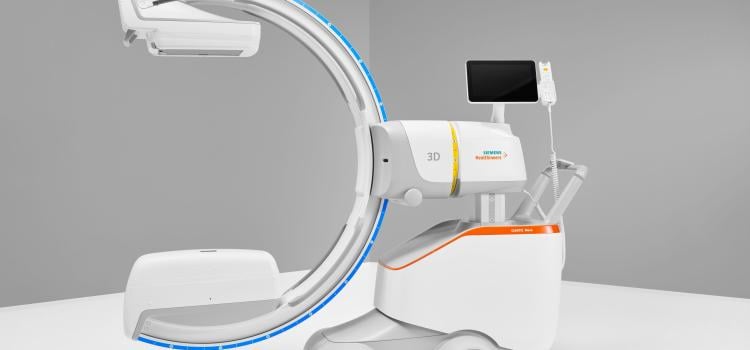
March 21, 2024 — Siemens Healthineers announces the Food and Drug Administration (FDA) clearance of the CIARTIC Move, a ...
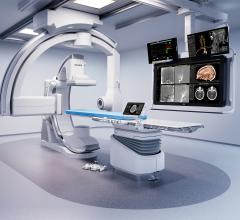
February 28, 2024 — Royal Philips, a global leader in health technology, announced major enhancements to its Image ...
July 29, 2022 — Siemens Healthineers has announced the Food and Drug Administration (FDA) clearance of the ARTIS icono ...
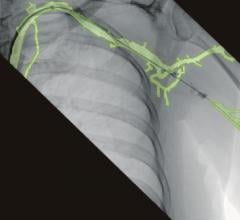
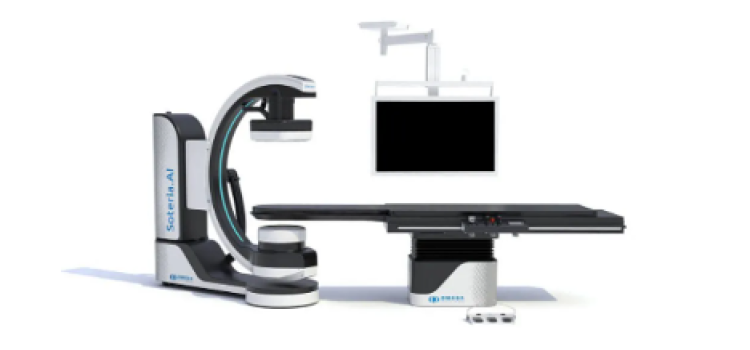
April 27, 2022 – Omega Medical Imaging has announced the release of the revolutionary Soteria.AI. Designed specifically ...
January 18, 2022 – Philips Healthcare announced physicians will now have access to advanced new 3D image guidance ...

January 19, 2021 – With the postponement of non-essential elective surgeries and medical procedures in 2020 to conserve ...
August 18, 2020 — Ziehm Imaging announces the acquisition of Therenva, a French-based developer of planning and imaging ...
September 17, 2019 — The U.S. Food and Drug Administration (FDA) has cleared the Artis icono, a high-precision family of ...
July 24, 2019 — The West Virginia University (WVU) Heart and Vascular Institute is the first hospital in the country to ...
May 23, 2019 — ControlRad Inc. announced that the U.S. Food and Drug Administration (FDA) granted 510(k) clearance for ...
February 18, 2019 — Philips announced the launch of Philips Zenition, its new mobile C-arm imaging platform. Mobile C ...
February 6, 2019 — Canon Medical Systems USA Inc. recently introduced a new angiography configuration featuring its ...
November 6, 2018 — Siemens Healthineers announced the U.S. Food and Drug Administration (FDA) clearance of the Cios Spin ...
January 9, 2017 — Whale Imaging Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the G-Arm Duo ...

 June 20, 2024
June 20, 2024