March 28, 2014 — St. Jude Medical Inc. announced the global launch of the Optisure defibrillation lead. This new lead is ...
Implantable Cardioverter Defibrillator (ICD)
This channel includes news and new technology innovations for implantable cardioverter defibrillators (ICD) used to treat tachycardia arrhythmias and heart failure. This includes cardiac resynchronization therapy defibrillators (CRT-D).

March 25, 2014 — Biotronik has announced the U.S. Food and Drug Administration (FDA) has approved the expansion of ...

October 14, 2013 — An independent study from the University of Pittsburgh Medical Center, published online this week in ...
October 10, 2013 — Biotronik announced the launch of the new Idova 7 series. Idova 7 ICDs (implantable cardioverter ...
October 9, 2013 — The U.S. Food and Drug Administration's (FDA) Circulatory Systems Devices Advisory Panel this week ...
October 3, 2013 — Devices like pacemakers and defibrillators allow doctors to introduce electrical signals to set ...
October 1, 2013 — The U.S. Food and Drug Administration (FDA) approved Biotronik’s Ilesto family of implantable ...
September 26, 2013 – Biotronik announced the highly-anticipated results of the Reform study. The study demonstrates that ...

September 25, 2013 – The U.S. Food and Drug Administration (FDA) announced a final rule for the unique device ...
September 13, 2013 – The U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices ...
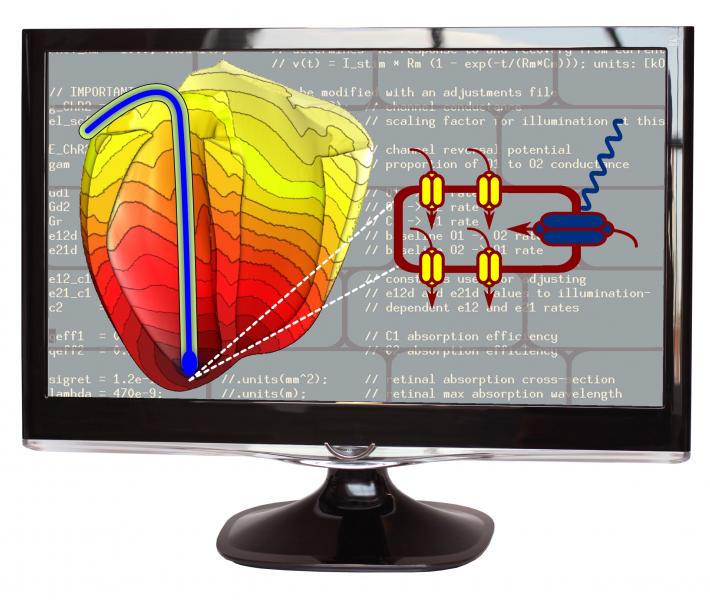
September 6, 2013 — When a beating heart slips into an irregular, life-threatening rhythm, the treatment is well known ...


 March 28, 2014
March 28, 2014