September 13, 2013 – The U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee will review two new technologies to help treat heart failure (HF) Oct. 8-9, 2013.
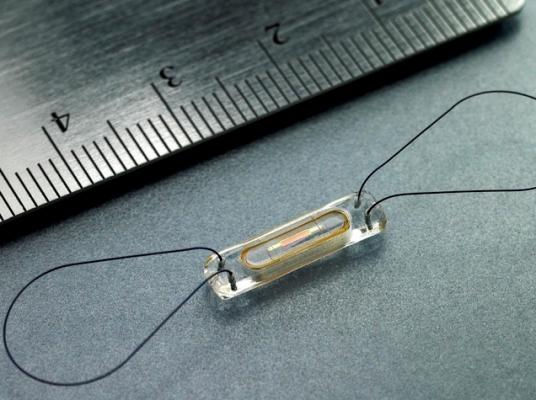
On Oct. 9, the committee will discuss, make recommendations and vote on information related to the premarket approval application for CardioMEMS Inc. Champion HF monitoring system. The system is a permanently implantable pressure measurement system designed to provide daily pulmonary arterial pressure measurements including systolic, diastolic and mean pulmonary arterial (PA) pressure. These measurements are used to guide treatment of congestive heart failure.
The system consists of an implantable sensor containing a three- dimensional coil and pressure-sensitive capacitor encased between two wafers of fused silica. The coil (inductor) electromagnetically couples to the sensor and allows the remote measurement of the resonant frequency of the inductive/capacitive circuit. This allows for wireless communication with the sensor and eliminates the need for an onboard source of energy, such as a battery.
The device is delivered within the distal pulmonary artery. There are two versions of the delivery system. The first includes a hydrophilic coating on the distal portion of the catheter shaft and the second has no coating on the catheter shaft. The delivery system (with HF sensor) is introduced over a guidewire through a sheath. Tether wires connect the sensor to the delivery system until the physician determines that the sensor is properly positioned within the distal pulmonary artery. Once the sensor is in position, the tether wires are withdrawn, releasing the sensor.
The electronics unit (interrogator) and database contain hardware and software to acquire and process signals from the sensor, provides a system interface for both patients and clinicians, and transfers PA measurements to a database for review by medical professionals. The database is a Web-based server that contains software, which receives data transmitted from the electronics unit, and presents the data for review by medical professionals.
On Oct. 8, the committee will review and make recommendations related to the expansion of heart failure indications to all market-approved Medtronic cardiac resynchronization therapy-pacemaker and cardiac resynchronization therapy-defibrillator devices.
The requested expansion in indications for use was studied under the Block HF trial. The trial was a prospective, multisite, randomized, double-blinded, parallel-controlled investigational device exemption study. The primary objective of the trial was to demonstrate that the time until the first event of all-cause mortality, heart-failure- related urgent care, or a significant increase in left ventricular end systolic volume index for subjects programmed to biventricular pacing is superior to that of subjects programmed to right ventricular pacing.
The meeting will be held each day from 8 a.m. to 6 p.m. at the Hilton Washington, D.C., North/Gaithersburg, Salons A-D, 620 Perry Pkwy., Gaithersburg, Md.
For more information: www.fda.gov/AdvisoryCommittees/Calendar/default.htm


 January 13, 2026
January 13, 2026 









