May 21, 2015 — Clinical trial results showed that full-body magnetic resonance imaging (MRI) scans do not affect the ...
Implantable Cardioverter Defibrillator (ICD)
This channel includes news and new technology innovations for implantable cardioverter defibrillators (ICD) used to treat tachycardia arrhythmias and heart failure. This includes cardiac resynchronization therapy defibrillators (CRT-D).
May 19, 2015 — A new epicardial pacing lead has been cleared by the U.S. Food and Drug Administration (FDA) as an option ...

May 15, 2015 — A new expert consensus statement by the Heart Rhythm Society (HRS) recommends remote monitoring (RM) beco ...
May 15, 2015 — Geneva Healthcare will showcase a set of new features on The Geneva Healthcare Suite – a technology ...
May 15, 2015 — St. Jude Medical Inc. announced CE Mark approval of expanded labeling for its Ellipse implantable ...
May 15, 2015 — Accreditation for Cardiovascular Excellence (ACE) has just released the first-ever electrophysiology (EP) ...
May 11, 2015 — Boston Scientific Corp. presented an overview of its continued business momentum and long-term growth ...
May 8, 2015 — The ScottCare Corp. announced it will highlight Ambucor, the company’s employable labor service division ...
April 27, 2015 — Biotronik announced that the U.S. Food and Drug Administration (FDA) has approved its DX line of ...
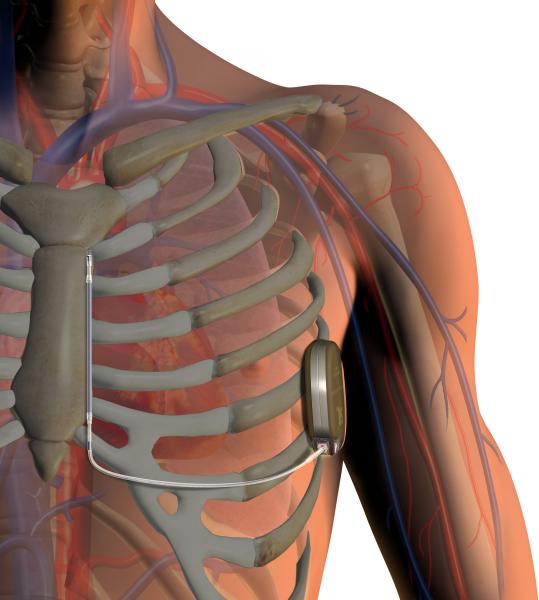
April 21, 2015 — The Journal of the American College of Cardiology published data confirming the long-term safety and ...
Learn about the trends and new technology in electrophysiology (EP) in an interview with David Wilber, M.D., editor of ...
March 19, 2015 — Boston Scientific Corp. has received U.S. Food and Drug Administration (FDA) and CE Mark approval of ...
March 5, 2015 – Biotronik announced that its ProMRI and ProMRI AFFIRM studies have been published in Heart Rhythm, the ...
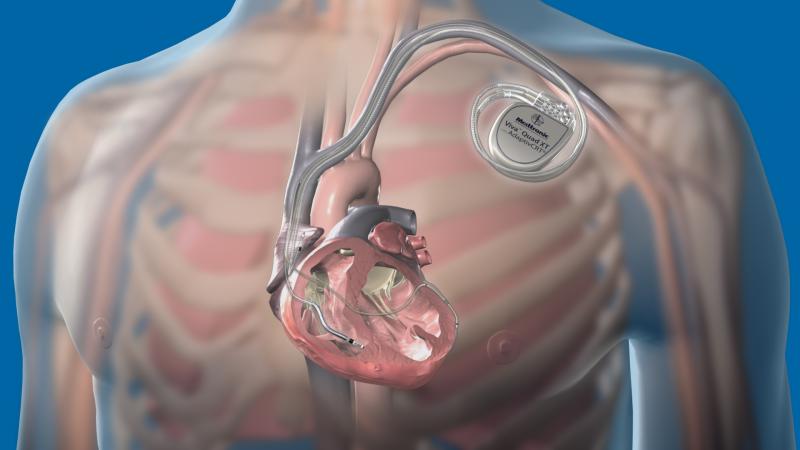
There have been several recent advancements in implantable cardioverter defibrillator (ICD) technology to extend battery ...

 May 21, 2015
May 21, 2015