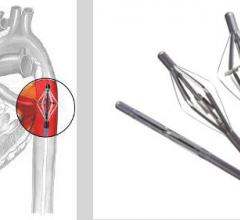
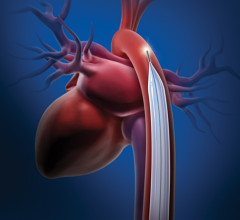
Since the 1970s, intra-aortic balloon pumps (IABPs) have been the gold standard of minimally invasive hemodynamic ...
Hemodynamic Support Devices
This hemodynamic support systems channel includes content on intra-aortic balloon pumps (IABP), percutaneous ventricular assist devices (pVAD) like the Impella or TandemHeart, extracorporeal membrane oxygenation (ECMO), and ventricular assist devices (VAD). This channel also includes use of these devices in support of patients in cardiogenic shock and advanced heart failure.
March 27, 2013 — The Heart Failure 2013 LA meeting will include a showcase of several startup companies offering ...
March 13, 2013 — CircuLite Inc. announced it has received conditional approval from the U.S. Food and Drug ...
My job as editor of DAIC is to help readers keep tabs on the latest in cardiovascular technology and trends. With ...
December 19, 2012 — CardiacAssist Inc. announced it has received investigational device exemption (IDE) approval from ...
December 17, 2012 — Abiomed Inc. announced the U.S. Food and Drug Administration's (FDA) Circulatory System Devices ...
Two key news items in September made me think about the future direction of hemodynamic support, and that intra-aortic ...

Two key news items in September made me think about the future direction of hemodynamic support, and that intra-aortic ...
December 6, 2012 — The U.S. Food and Drug Administration (FDA) granted premarket approval (PMA) for the HeartWare ...
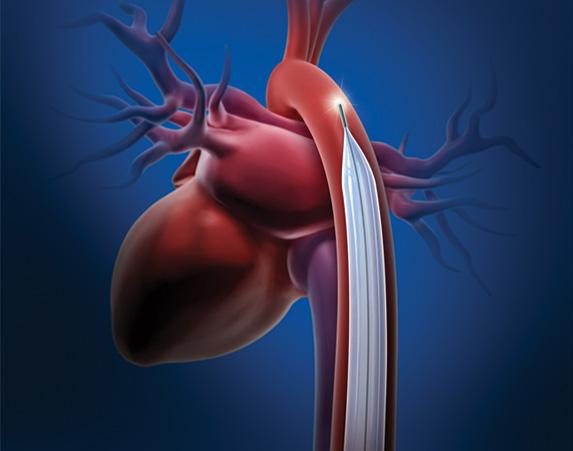
November 8, 2012 — A U.S. Food and Drug Administration (FDA) advisory committee is meeting Dec. 5 to discuss how to ...
October 30, 2012 — Maquet Cardiovascular received 510(k) clearance from the U.S. Food and Drug Administration (FDA) and ...
October 3, 2012 — The National Institutes of Health (NIH)/National Cancer Institute (NCI) has awarded a five-year ...
October 1, 2012 — CircuLite Inc. announced that the latest data from patients enrolled in the company’s CE mark trial of ...
September 18, 2012 — Abiomed Inc. announced it received confirmation from the American Medical Association (AMA) of ...
CircuLite Inc. announced that it has received CE Marking approval for the Synergy Circulatory Support System, a micro ...


 May 13, 2013
May 13, 2013