Behnam Tehrani, M.D., FSCAI, director of the cardiac cath lab, INOVA Heart and Vascular Institute, Fairfax, Va ...
Hemodynamic Support Devices
This hemodynamic support systems channel includes content on intra-aortic balloon pumps (IABP), percutaneous ventricular assist devices (pVAD) like the Impella or TandemHeart, extracorporeal membrane oxygenation (ECMO), and ventricular assist devices (VAD). This channel also includes use of these devices in support of patients in cardiogenic shock and advanced heart failure.
July 17, 2018 — Medtronic plc recently received U.S. Food and Drug Administration (FDA) approval for a less-invasive ...
July 11, 2018 — French-based CorWave announced that its CALYPSO program has received 14 million euros to develop CorWave ...


About 50 percent of patients in cardiogenic shock do not survive, and account for about 90,000 heart attack patients a ...
June 6, 2018 — Maquet Datascope Corp. said it is recalling the CardioSave Hybrid Intra-aortic Balloon Pump (IABP) due to ...
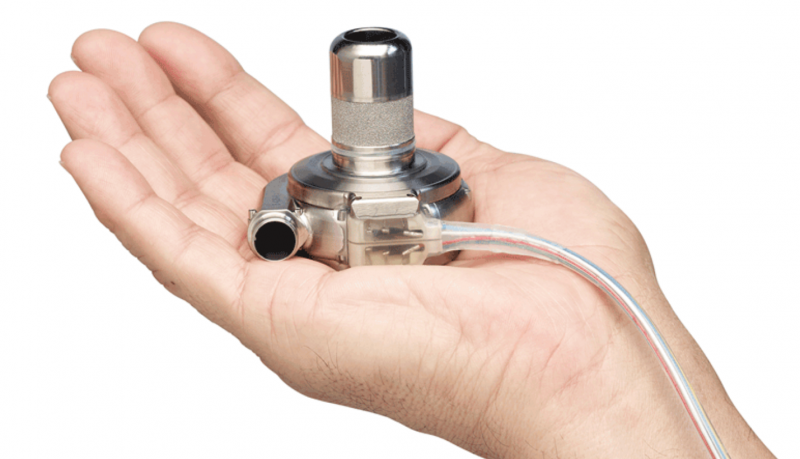
June 4, 2018 — The U.S. Food and Drug Administration said Medtronic initiated a Class 1 recall all 204,017 of its ...

May 31, 2018 — The results from the Survival Improvement in Extensive Myocardial Infarction with PERsistent Ischemia ...
May 23, 2018 — Abbott has initiated a Class I recall of the HeartMate 3 Left Ventricular Assist System due to a ...
May 2, 2018 — Select adult patients born with a single functioning ventricle, and who have undergone a surgical ...
April 12, 2018 – French medical technology company CorWave announced it has been awarded almost $3.5 million in ...
April 2, 2018 — Abiomed Inc. announced that it received U.S. Food and Drug Administration (FDA) Pre-Market Approval (PMA ...
David Lanfear M.D., FACC, head of advanced heart failure and cardiac transplantation, Henry Ford Hospital, Detroit ...
March 13, 2018 — At two years of follow-up, severely ill patients with advanced heart failure who received a novel heart ...
This video details the first use of a new protocol at The Ohio State University’s Wexner Medical Center to start sudden ...


 August 31, 2018
August 31, 2018