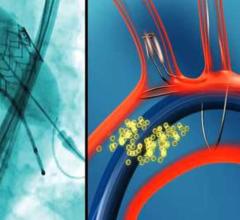
November 9, 2016 — Patients who undergo minimally invasive heart valve replacement, known as transcatheter aortic valve ...
Heart Valve Technology
This channel includes news and new device innovations about heart valve technologies, including the aortic valve, mitral valve, pulmonic valve, and tricuspid valve. This includes information on transcatheter valve technologies like transcatheter aortic valve replacement (TAVR, or implantation TAVI), transcatheter mitral valve repair or replacement (TMVR), transcatheter and surgical valve repairs, and surgical replacement valves. Newer devices are now being used for transcatheter tricuspid valve repair replacement (TTVR).
Studies have shown transcatheter aortic valve replacement (TAVR) has an increased risk of stroke and cerebral damage due ...
November 7, 2016 — Edwards Lifesciences Corp. recently announced new data demonstrating dramatic and sustained ...
November 7, 2016 — Keystone Heart Ltd. announced that data presented at the Transcatheter Cardiovascular Therapeutics ...
November 7, 2016 – A multicenter randomized trial evaluating the role of embolic protection using the Sentinel device ...

November 4, 2016 — Here is the list of the top 20 most popular pieces of content on the Diagnostic and Interventional ...
November 2, 2016 — Medtronic plc recently announced new clinical data for the Harmony Transcatheter Pulmonary Valve (TPV ...
November 1, 2016 — Edwards Lifesciences Corp. announced new five-year hemodynamic data from the PARTNER Trial ...
October 31, 2016 — Medtronic plc presented new positive data from two large registries aimed at evaluating 30-day ...
October 28, 2016 — Mitralign will present data on its Trialign Tricuspid Repair system at the 2016 Transcatheter ...

The volume of information attendees receive at the annual Transcatheter Cardiovascular Therapeutics (TCT) interventional ...
October 27, 2016 — BioTrace Medical Inc. said it received U.S. Food and Drug Administration (FDA) 510(k) clearance for ...
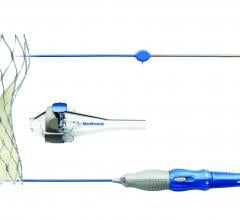
October 26, 2016 — Medtronic plc announced the U.S. Food and Drug Administration (FDA) approval and U.S. launch of the ...
October 10, 2016 — Francesco Maisano, clinic director at the University Hospital Zurich, recently led a team of cardiac ...
October 7, 2016 — A Northwestern Medicine cardiac surgeon was recently the first in Illinois and second in the United ...

 November 09, 2016
November 09, 2016